$Takeda Pharmaceutical(TAK.US$ FDA had approved yoohooo
2
1
@Ardene @chgocal123 @ConchTrader @Daniel Acret @Edwin SG @KS_TAN @Lee Jessie @Lin Bao Bao @Machiavellis3rdEye @Michael_Lawrence @Mike Hunt @Mr Trecherous @OldNormanBates @ong ah boy @Revelations 6 @Tanya C @Technical DNA @Tupack H Mcsnacks @treydongui
$Takeda Pharmaceutical(TAK.US$ Tomorrow 23/11/21 expecting PDUFA approval for Maribavir.
About Maribavir
Maribavir, an orally bioavailable anti-CMV compound, is the only antiviral agent presently in Phase 3 development for the treatment of post-transplant patients with CMV in SOT or HCT. Maribavir is an investigational treatment that has not been approved for use by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) or any other regulatory authorities. Maribavir is the only CMV antiviral drug that targets and inhibits the UL97 protein kinase and its natural substrates.15-18
Maribavir has been granted Orphan Drug Designation by the European Commission as a treatment of CMV disease in patients with impaired cell mediated immunity and by the FDA for treatment of clinically significant CMV viremia and disease in at-risk patients. Orphan status is granted to certain investigational medicines intended for the treatment or prevention of a rare, life-threatening disease. The FDA has also granted maribavir Breakthrough Therapy Designation as a treatment for CMV infection and disease in transplant patients resistant or refractory to prior therapy. Breakthrough Therapy Designation expedites the development and review of investigational treatments for serious conditions with preliminary clinical evidence indicating that the drug may demonstrate substantial improvement over available therapy. These designations do not guarantee that the EMA or FDA will approve maribavir for the treatment of CMV infections in transplant patients, and the timing of any such approval is uncertain.
About Takeda’s SOLSTICE Trial
The TAK-620-303 (SOLSTICE) trial (NCT02931539) is a multicenter, randomized, open-label, active-controlled trial comparing eight weeks of treatment with either maribavir or investigator assigned treatment, IAT, (conventional antiviral therapy) in hematopoietic cell transplant and solid organ transplant recipients with CMV infection refractory, with or without resistance, to one or a combination of the conventional antiviral therapies: ganciclovir, valganciclovir, foscarnet or cidofovir. Patients underwent a 2-week screening period, followed by randomization 2:1 to maribavir (n=235) (400 mg) or IAT (n=117) for an 8-week treatment period, plus 12 weeks of follow-up.
The trial’s primary endpoint was defined as the proportion of patients who achieved confirmed CMV viremia clearance (plasma CMV DNA <137 IU/mL in two consecutive tests ≥5 days apart at central laboratory) compared to IAT at the end of Study Week 8. The key secondary endpoint was defined as achievement of CMV viremia clearance and symptom control at end of Study Week 8, maintained through Study Week 16.
Takeda's Maribavir Phase 3 Clinical Trial Met Primary Endpoint of Superiority to Conventional Antiviral Therapy in Transplant Recipients With Refractory, With or Without Resistance, Cytomegalovirus Infection/Disease
$Takeda Pharmaceutical(TAK.US$ Tomorrow 23/11/21 expecting PDUFA approval for Maribavir.
About Maribavir
Maribavir, an orally bioavailable anti-CMV compound, is the only antiviral agent presently in Phase 3 development for the treatment of post-transplant patients with CMV in SOT or HCT. Maribavir is an investigational treatment that has not been approved for use by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) or any other regulatory authorities. Maribavir is the only CMV antiviral drug that targets and inhibits the UL97 protein kinase and its natural substrates.15-18
Maribavir has been granted Orphan Drug Designation by the European Commission as a treatment of CMV disease in patients with impaired cell mediated immunity and by the FDA for treatment of clinically significant CMV viremia and disease in at-risk patients. Orphan status is granted to certain investigational medicines intended for the treatment or prevention of a rare, life-threatening disease. The FDA has also granted maribavir Breakthrough Therapy Designation as a treatment for CMV infection and disease in transplant patients resistant or refractory to prior therapy. Breakthrough Therapy Designation expedites the development and review of investigational treatments for serious conditions with preliminary clinical evidence indicating that the drug may demonstrate substantial improvement over available therapy. These designations do not guarantee that the EMA or FDA will approve maribavir for the treatment of CMV infections in transplant patients, and the timing of any such approval is uncertain.
About Takeda’s SOLSTICE Trial
The TAK-620-303 (SOLSTICE) trial (NCT02931539) is a multicenter, randomized, open-label, active-controlled trial comparing eight weeks of treatment with either maribavir or investigator assigned treatment, IAT, (conventional antiviral therapy) in hematopoietic cell transplant and solid organ transplant recipients with CMV infection refractory, with or without resistance, to one or a combination of the conventional antiviral therapies: ganciclovir, valganciclovir, foscarnet or cidofovir. Patients underwent a 2-week screening period, followed by randomization 2:1 to maribavir (n=235) (400 mg) or IAT (n=117) for an 8-week treatment period, plus 12 weeks of follow-up.
The trial’s primary endpoint was defined as the proportion of patients who achieved confirmed CMV viremia clearance (plasma CMV DNA <137 IU/mL in two consecutive tests ≥5 days apart at central laboratory) compared to IAT at the end of Study Week 8. The key secondary endpoint was defined as achievement of CMV viremia clearance and symptom control at end of Study Week 8, maintained through Study Week 16.
Takeda's Maribavir Phase 3 Clinical Trial Met Primary Endpoint of Superiority to Conventional Antiviral Therapy in Transplant Recipients With Refractory, With or Without Resistance, Cytomegalovirus Infection/Disease

5
$CorMedix(CRMD.US$
For those who wants a quick buck, probably this is not the place for you now.
This drugs might have the same potential as CCXI Avacopan. Company have address their shortcomings and now only need to submit NDA to FDA whereby no timeline was given yet.
Company submission for NDA was rejected by FDA previously, as there was some issues at the third-party manufacturer, or CMO, manufacture of DefenCath.
The Company received a Complete Response Letter from FDA stating that the NDA could not be approved until satisfactory resolution of deficiencies at the contract manufacturing facility, including in-process controls for the filling operation.
Together with our CMO, we have been able to resume manufacturing activities and are continuing to complete the work that is required to address the deficiencies identified at the manufacturing facility.
Dr. Matt David, CorMedix interim CEO, commented, “We are pleased that we have been able to resume manufacturing activities at our CMO and look forward to providing updates over the coming months. As the recent industry conference presentations have highlighted, catheter related bloodstream infections are common in patients receiving hemodialysis via central venous catheters and are associated with significant morbidity and mortality. We remain steadfast in our commitment to these patients as we seek to bring DefenCath to market upon its approval.” CorMedix continues to work diligently toward the resubmission of the DefenCath New Drugs Application and plans to provide an update when we have clarity on the submission timeline.
6. DefenCath has been designated by FDA as Fast Track and as a Qualified Infectious Disease Product (QIDP), and the NDA received priority review in recognition of its potential to address an unmet medical need. QIDP provides for an additional five years of marketing exclusivity, which will be added to the five years granted to a New Chemical Entity upon approval of the NDA. CorMedix also committed to conducting a clinical study in pediatric patients using a central venous catheter for hemodialysis when the NDA is approved, which will add an additional six months of marketing exclusivity when the study is completed.
For those who wants a quick buck, probably this is not the place for you now.
This drugs might have the same potential as CCXI Avacopan. Company have address their shortcomings and now only need to submit NDA to FDA whereby no timeline was given yet.
Company submission for NDA was rejected by FDA previously, as there was some issues at the third-party manufacturer, or CMO, manufacture of DefenCath.
The Company received a Complete Response Letter from FDA stating that the NDA could not be approved until satisfactory resolution of deficiencies at the contract manufacturing facility, including in-process controls for the filling operation.
Together with our CMO, we have been able to resume manufacturing activities and are continuing to complete the work that is required to address the deficiencies identified at the manufacturing facility.
Dr. Matt David, CorMedix interim CEO, commented, “We are pleased that we have been able to resume manufacturing activities at our CMO and look forward to providing updates over the coming months. As the recent industry conference presentations have highlighted, catheter related bloodstream infections are common in patients receiving hemodialysis via central venous catheters and are associated with significant morbidity and mortality. We remain steadfast in our commitment to these patients as we seek to bring DefenCath to market upon its approval.” CorMedix continues to work diligently toward the resubmission of the DefenCath New Drugs Application and plans to provide an update when we have clarity on the submission timeline.
6. DefenCath has been designated by FDA as Fast Track and as a Qualified Infectious Disease Product (QIDP), and the NDA received priority review in recognition of its potential to address an unmet medical need. QIDP provides for an additional five years of marketing exclusivity, which will be added to the five years granted to a New Chemical Entity upon approval of the NDA. CorMedix also committed to conducting a clinical study in pediatric patients using a central venous catheter for hemodialysis when the NDA is approved, which will add an additional six months of marketing exclusivity when the study is completed.
19
4
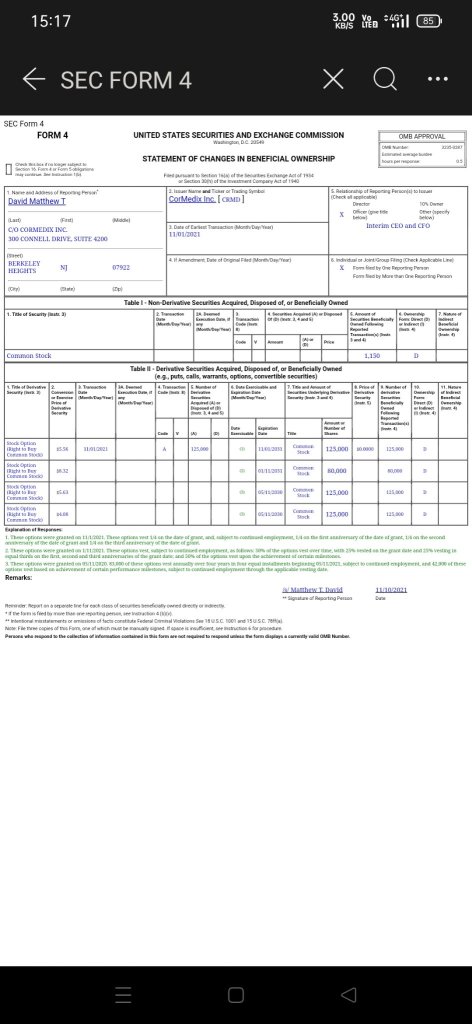
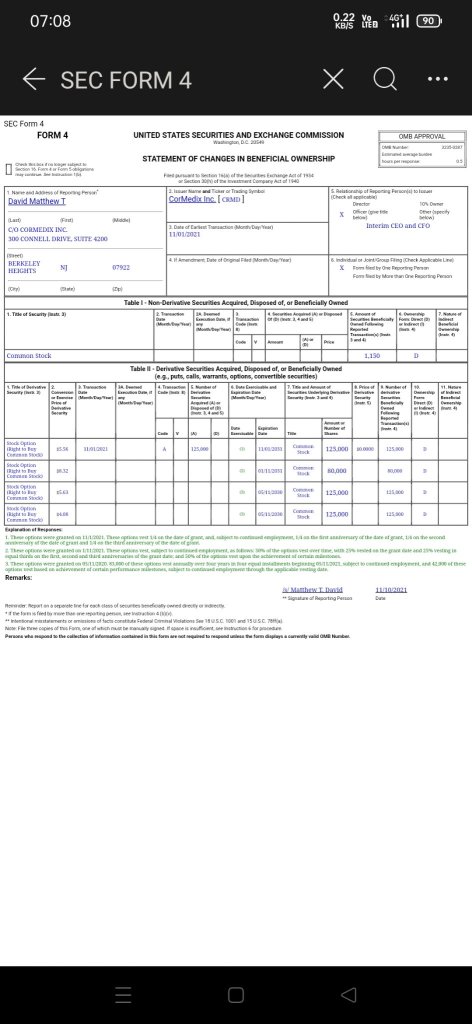
$CorMedix(CRMD.US$ More clarity on the directions and progress.
1. CEO bought 125k shares recently.
2. Company provided an update that as noted previously, there was a delay as a result of issues at the third-party manufacturer, or CMO, unrelated to the manufacture of DefenCath. Together with our CMO, we have been able to resume manufacturing activities and are continuing to complete the work that is required to address the deficiencies identified at the manufacturing facility.
3. CorMedix presented three abstracts at industry conferences including the Association of Managed Care Pharmacy (AMCP) Nexus conference in October and the American Society of Nephrology (ASN) conference in November. The presentations highlighted retrospective analyses that were conducted to better understand the incidence of and mortality related to CRBSIs and economic costs related to these infections.
4. Dr. Matt David, CorMedix interim CEO, commented, “We are pleased that we have been able to resume manufacturing activities at our CMO and look forward to providing updates over the coming months. As the recent industry conference presentations have highlighted, catheter related bloodstream infections are common in patients receiving hemodialysis via central venous catheters and are associated with significant morbidity and mortality. We remain steadfast in our commitment to these patients as we seek to bring DefenCath to market upon its approval.”
5. CorMedix continues to work diligently toward the resubmission of the DefenCath New Drugs Application and plans to provide an update when we have clarity on the submission timeline.
6. DefenCath has been designated by FDA as Fast Track and as a Qualified Infectious Disease Product (QIDP), and the NDA received priority review in recognition of its potential to address an unmet medical need. QIDP provides for an additional five years of marketing exclusivity, which will be added to the five years granted to a New Chemical Entity upon approval of the NDA. CorMedix also committed to conducting a clinical study in pediatric patients using a central venous catheter for hemodialysis when the NDA is approved, which will add an additional six months of marketing exclusivity when the study is completed. The Company received a Complete Response Letter from FDA stating that the NDA could not be approved until satisfactory resolution of deficiencies at the contract manufacturing facility, including in-process controls for the filling operation. CorMedix also intends to develop DefenCath as a catheter lock solution for use in oncology and total parenteral nutrition patients. It is leveraging its taurolidine technology to develop a pipeline of antimicrobial medical devices, with programs in surgical sutures and meshes, and topical hydrogels. The Company is also working with top-tier researchers to develop taurolidine-based therapies for rare pediatric cancers. Neutrolin® is CE Marked and marketed in Europe and other territories as a medical device.
There's no time line given at the moment.
1. CEO bought 125k shares recently.
2. Company provided an update that as noted previously, there was a delay as a result of issues at the third-party manufacturer, or CMO, unrelated to the manufacture of DefenCath. Together with our CMO, we have been able to resume manufacturing activities and are continuing to complete the work that is required to address the deficiencies identified at the manufacturing facility.
3. CorMedix presented three abstracts at industry conferences including the Association of Managed Care Pharmacy (AMCP) Nexus conference in October and the American Society of Nephrology (ASN) conference in November. The presentations highlighted retrospective analyses that were conducted to better understand the incidence of and mortality related to CRBSIs and economic costs related to these infections.
4. Dr. Matt David, CorMedix interim CEO, commented, “We are pleased that we have been able to resume manufacturing activities at our CMO and look forward to providing updates over the coming months. As the recent industry conference presentations have highlighted, catheter related bloodstream infections are common in patients receiving hemodialysis via central venous catheters and are associated with significant morbidity and mortality. We remain steadfast in our commitment to these patients as we seek to bring DefenCath to market upon its approval.”
5. CorMedix continues to work diligently toward the resubmission of the DefenCath New Drugs Application and plans to provide an update when we have clarity on the submission timeline.
6. DefenCath has been designated by FDA as Fast Track and as a Qualified Infectious Disease Product (QIDP), and the NDA received priority review in recognition of its potential to address an unmet medical need. QIDP provides for an additional five years of marketing exclusivity, which will be added to the five years granted to a New Chemical Entity upon approval of the NDA. CorMedix also committed to conducting a clinical study in pediatric patients using a central venous catheter for hemodialysis when the NDA is approved, which will add an additional six months of marketing exclusivity when the study is completed. The Company received a Complete Response Letter from FDA stating that the NDA could not be approved until satisfactory resolution of deficiencies at the contract manufacturing facility, including in-process controls for the filling operation. CorMedix also intends to develop DefenCath as a catheter lock solution for use in oncology and total parenteral nutrition patients. It is leveraging its taurolidine technology to develop a pipeline of antimicrobial medical devices, with programs in surgical sutures and meshes, and topical hydrogels. The Company is also working with top-tier researchers to develop taurolidine-based therapies for rare pediatric cancers. Neutrolin® is CE Marked and marketed in Europe and other territories as a medical device.
There's no time line given at the moment.
10
2
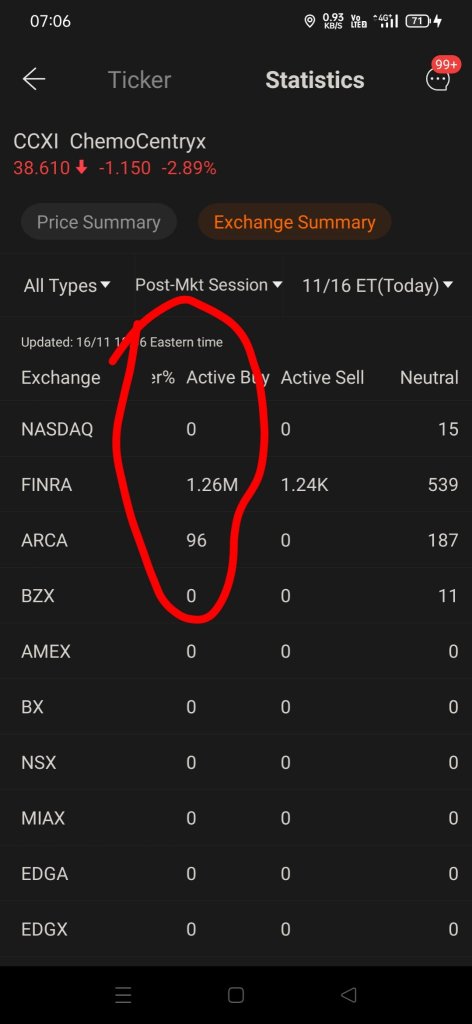
$ChemoCentryx(CCXI.US$ guys hold on to the moon today might be the day HF and options call (expiring 19/11/21) (exercising their option to buy ) look at the post market 1.26million buy in. If I'm not wrong base on my observation, yesterday HF did 3 rounds of shorting. 1st was when market open but they manage to cover back, the price drop to $38.58 and climb back to $39.99. because base on daily short interest ratio it's between 1.2-1.3. so that means the 2nd and 3rd round of shorting wasn't cover by them yet hence post market there's a put up of 1.26m buy in. Today there's also the investor conference by CCXI. Latest Friday the price will uptrend. Please don't panic sell and lose your chance of earning more.
10
2
$CorMedix(CRMD.US$
CEO cum CFO bought shares, it's a good sign. Everyone wants a share of the pie. Good luck 😃🤞
CEO cum CFO bought shares, it's a good sign. Everyone wants a share of the pie. Good luck 😃🤞
$ChemoCentryx(CCXI.US$ During my previous post I mentioned that CCXI COMP meeting (discussion) was from 3-5 November and CHMP meetings (adoption) on 08-11 November.
Today they will conclude their meeting and base on last month CHMP
October 2021 meeting, EMA consolidated and give an update on the minutes of meeting the very next day.
I presume the Company would have known the outcome and will be announcing anytime. So hang on to your shares or you might miss a huge reward.
Today they will conclude their meeting and base on last month CHMP
October 2021 meeting, EMA consolidated and give an update on the minutes of meeting the very next day.
I presume the Company would have known the outcome and will be announcing anytime. So hang on to your shares or you might miss a huge reward.
18
1
![[empty]](https://static.moomoo.com/node_futunn_nnq/assets/images/folder.5c37692712.png)
![[error]](https://static.moomoo.com/node_futunn_nnq/assets/images/no-network.991ae8055c.png)
Dennis Takizawa HideOP :