- For printing
- May 22, 2024
Eisai Co., Ltd
Nippon Medac Co., Ltd.
Eisai Co., Ltd. (Headquarters: Tokyo; Representative Executive Officer and CEO: Naito Haruo, hereafter Eisai) and Medac GmbH (Headquarters: Germany), Nippon Medac Co., Ltd. (Headquarters: Tokyo; President: Iriyama Hirohisa; hereafter, Nippon Medac) announced today in Japan the automatic injector injector injection “metjector for subcutaneous injection of methject, which is an antirheumatic agentI would like to inform you that “subcutaneous 7.5 mg pen 0.15 ml, 10 mg pen 0.20 ml, same 12.5 mg pen 0.25 ml, same 15 mg pen 0.30 ml” (generic name: methotrexate (MTX)) have been newly released. This drug was approved for manufacture and sale on 2024/2/15, and today, the drug price was listed. Based on the license agreement between Eisai and Medac GmbH in 2019/5, Nippon Medac holds approval for the manufacture and sale of this drug, and Eisai is responsible for sales.
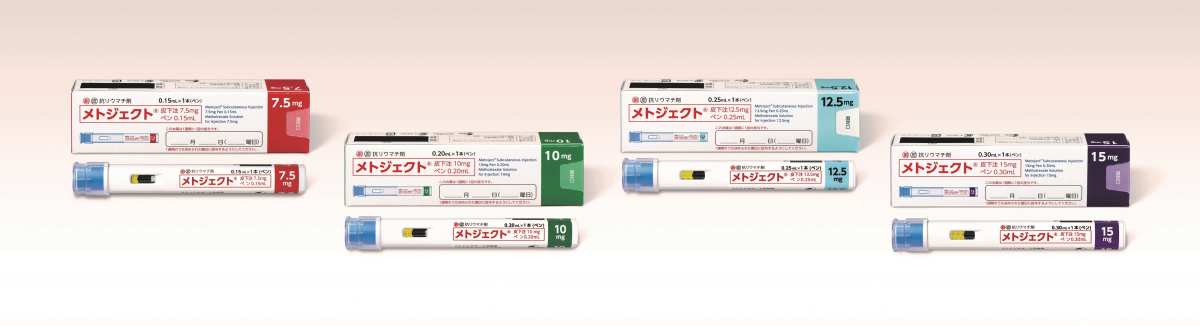
The number of rheumatoid arthritis patients in Japan is estimated to be around 700,000 to 800,000, and MTX is used as a first-line drug in rheumatoid arthritis treatment1. This drug is the first self-administered MTX pen-type automatic injector injection in Japan for rheumatoid arthritis, and was developed for the purpose of reducing the burden on patients during self-injection and improving safety. This drug can be self-injected in 2 steps (① remove the cap, ② press against the skin). Also, since the needle cover is built in, the needle cannot be seen before administration, and the risk of incorrect puncture is reduced by automatically locking the needle cover even after administration.
Eisai Japan President Naoki Sawada said, “Eisai has built a leading franchise in the rheumatoid arthritis area in Japan and has extensive sales experience. By adding this drug to Eisai's product lineup, we will meet the diverse needs of rheumatoid arthritis patients and further contribute by improving benefits.”
Hirohisa Iriyama, President and CEO of Nippon Medac, said, “By providing metoject subcutaneous injection pens as a new treatment option for rheumatoid arthritis patients, safe, and easy self-injections are expected. Nippon Medac will continue to contribute to the treatment of rheumatoid arthritis that is close to patients.”
over
For press inquiries regarding this matter, please contact
-
Eisai Co., Ltd
PR department
TEL:03-3817-5120
-
Nippon Medac Co., Ltd.
Person in charge: Otsuki
TEL:03-6661-6270
-
Contact medical personnel for inquiries regarding this matter
Eisai hhchotlines
TEL: 0120-419-497
Reception: 9:00 to 18:00 on weekdays,
Available 365 days a year from 9:00 to 17:00 on weekends and holidays
-
Contact information for inquiries about this drug
Eisai hhchotlines
TEL: 0120-151-454
Reception: 9:00 to 18:00 on weekdays,
Available 365 days a year from 9:00 to 17:00 on weekends and holidays
<Reference Materials>
1. Product Overview
Product name: MetjectSubcutaneous 7.5 mg pen 0.15 ml, same 10 mg pen 0.20 ml, same 12.5 mg pen 0.25 ml, same 15 mg pen 0.30 ml
Generic name: methotrexate
Efficacy or effect: rheumatoid arthritis
Usage and dosage: Usually, adults are injected subcutaneously with 7.5 mg as methotrexate once a week. Note that the dose can be increased as appropriate according to the patient's condition, tolerability, etc., but it should not exceed 15 mg.
Drug price: MetoJect Dermal Injection 7.5 mg pen 0.15 ml 1,938 yen/bottle
Same 10mg pen 0.20ml 2,310 yen/book
Same 12.5 mg pen 0.25 ml 2,652 yen/book
Same 15 mg pen 0.30 ml 2,972 yen/book
Packaging: Metoject subcutaneous 7.5 mg pen 0.15 ml 1 bottle
Same 10mg pen 0.20ml 1 bottle
1 bottle of the same 12.5mg pen 0.25ml
Same 15mg pen 0.30ml 1 bottle
- 2. About “Metject Dermal Injection Pen” (generic name: methotrexate)
Methotrexate (MTX) is positioned as an anchor drug for rheumatoid arthritis treatment2. Effects on rheumatoid arthritis include cytostatic effects due to inhibition of folic acid metabolism in lymphocytes, etc., and anti-inflammatory effects by promoting adenosine synthesis in vascular endothelial cells, etc. in synovial membranes. This drug is the first self-administered MTX pen type automatic injector injection in Japan targeting rheumatoid arthritis. Overseas, it has been approved in over 18 European countries.
- 3. About Eisai Co., Ltd.
Eisai Co., Ltd. puts the joys and sorrows of patients and consumers first, and has a corporate philosophy of “human health care (hhc),” which contributes to improving benefits, and based on this philosophy, it aims to efficiently realize the social good of “resolving health concerns” and “correcting medical care ranges.” We have a global R&D, production, and sales base network, and are working to create and provide innovative new drugs targeting diseases with high unmet medical needs centered on the “neurological area” and “cancer domain,” which are strategically important areas.
Furthermore, we are actively working with partners around the world on activities aimed at controlling “neglected tropical diseases (NTDs),” which is the target (3.3) of the United Nations Sustainable Development Goals (SDGs).
Detailed information on Eisai Co., Ltd.https://www.eisai.co.jpPlease see SNS accountX,LinkedIn,FacebookBut the information is open.
- 4. About Nippon Medac Co., Ltd.
Nippon Medac Co., Ltd. was established in April 2016 as a Japanese corporation of medac GmbH. We are working on the development of new pharmaceuticals that make it possible to expand treatment options for diseases where medical needs have not yet been sufficiently met. In order to improve the quality of life of patients and their families in areas with high medical needs, we will make diligent efforts to deliver high-quality drugs as soon as possible.
-
1
Report from Study Committee on Rheumatoid Arthritis and Allergy
http://www.mhlw.go.jp/stf/houdou/2r9852000001nfao-att/2r9852000001nfdx.pdf -
2
The Japanese Society of Rheumatology Methotrexate (MTX) Clinical Guidelines for Rheumatoid Arthritis 2016 Revised Edition
