Under the intertwined influence of the risk factors of globalization, how can enterprises persevere and continue to move forward in the midst of turbulence has become a common problem in the industry. However, Kingsley seemed to have given his own unique answer with an excellent questionnaire.
Recently, some media quoted foreign pharmaceutical industry media Endpoints as reporting that the “Biosafety Act” may be extended to more Chinese companies, including Kanglong Chemical, headquartered in Beijing, and Kingsley Biotechnology (“Kingsley” for short), headquartered in Nanjing. After the news came out, Kingsley Biotech's stock price fell sharply. To this end, the reporter consulted King's Rui as an investor. The other party said that currently there is no mention of King's Rui in the official versions of the Senate and House of Representatives “Biosafety Act,” and there are no official sources in the original foreign media reports.
The reporter then carried out a detailed analysis of the original text of the article and found that the article had many questions, and there was a suspicion that the two companies mentioned in the article were mistaken.
First, the article did not cite any official documents or sources. Currently, the official version of the Biosafety Bill in the Senate and House of Representatives is still being debated, and may never become law. Even if enacted, it's uncertain whether the bill's impact will extend to more companies.
Second, the author's original article only explains that the company is disclosing some of the possible risks from the perspective of corporate disclosure in the recent US earnings season. This behavior is similar to the risk warning for A-shares. However, the author has searched the vast majority of US listed companies. Only Calidi in the article gave risk alerts in financial disclosures; the vast majority of companies did not make similar disclosures. Finally, the article also emphasized many times that most US pharmaceutical companies will cause long-term difficulties and delays in their projects after the disorderly expansion of sanctions.
Wei Shiniu, chief financial officer of Kingsley, clarified in an interview with 21st Century Economic Report that although the US currently has many proposed laws, “none of them directly involves us,” and said that the company will continue to closely monitor related developments. Wei Shiniu also stressed that the relevant reports are only personal speculations.
Therefore, the author has reason to believe that the market had quite a misinterpretation of the Endpoints report, which induced instability in market sentiment, which in turn caused a round of mistaken killing of Kingsley's stock price. With the approval of CARVYKTI (Cilta-cel) of Legendary Biotech, a subsidiary of Kingsley, and the improvement in overall profitability after the release of Kingsley's results, we believe Kingsley is highlighting its huge investment value.
Next, please follow the author's perspective to fully examine the legendary King's Rui.
On April 5, 2024, US Eastern Time, Legend Biotech, a subsidiary of Kingsley, announced in Somerset, New Jersey, USA, that the US Food and Drug Administration has approved CARVYKTI (sidagiolense, cilta-cel) to treat patients with recurrent or refractory multiple myeloma. These patients have received at least first-line treatment in the past, including a proteasome inhibitor (PI) and an immunomodulator (IMID), and are resistant to lenalidomide. CARVYKTI is the first and only B-cell maturation antigen (BCMA) targeted therapy approved for second-line treatment of patients with multiple myeloma. It is expected that Sidakiol will also receive new commercialization approvals in Europe and China this year.
As of the first quarter of 2024, the cumulative sales volume of Sidaki Orense since its launch in the US was US$790 million, of which sales in 2023 exceeded US$500 million. According to the cooperation agreement with Johnson & Johnson, Johnson & Johnson will share costs and benefits at a ratio of 3:7 in Greater China; in the rest of the world, the agreed ratio is 5:5. According to Johnson & Johnson's 2023 Investor Open Day, the number of patients with multiple myeloma stage 4 and later is about 22,000, and the number of patients in line 2 & 3 is close to 118,000. After the second line is approved, CARVYKTI will benefit more patients around the world. At the same time, Johnson & Johnson expects that in 2027, CARVYKTI is expected to reach sales of about 3.6 billion US dollars, which is about 25% higher than market expectations. The journal “Nature” also published an article predicting that Sidakiol Orense will become the best-selling cell therapy in the world in 2027. Currently, Kingsley is fully integrated with the subsidiary Legendary Biotech. It is expected that as sales rise, the parent company Kingsley will usher in a concentrated harvest period for innovative drugs in the next two years.
In 2023, Kingsley showed a steady growth momentum by firmly advancing its global layout and strategic outlook. Revenue surged 34.2% year over year, and profitability rose steadily. Looking back at the development history of Kingsley, it is easy to see that its strategic deployment in various business segments is accurate and farsighted, and has always been at the forefront of the industry. Whether it is deeply involved in the mainland China market or winning the global stage, Kingsley has achieved remarkable results, and has also set a quality benchmark for the biotechnology industry in global segments.
So, what do you think of Kingsley's report card?
Revenue increased 34.2% year over year, and profitability gradually increased
In 2023, the company achieved revenue of US$840 million, an increase of 34.2% year on year; R&D expenditure of US$433 million, an increase of 10.95% year on year. Notably, the company did not sacrifice profit margin due to continued R&D investment. Overall gross profit was approximately US$410 million, an increase of 34.7% over the previous year.
Specifically, on the revenue side, the company showed impressive growth in several business segments, including external revenue from life science services and products business of US$405 million, up 15.6% year on year; external revenue from biopharmaceutical CDMO business of US$107 million; and external revenue from synthetic biology products of US$42.9 million, up 12.2% year on year. In addition, thanks to the rapid sales volume of CARVYKTI products in 2023, the company's external revenue in the field of cell therapy was US$285 million, an increase of 144.2% over the previous year, accounting for a rapid rise in total revenue from 14% in 2021 to 34% in 2023, showing strong market potential and commercialization capabilities.
On the profit side, the company's life science business has achieved profit growth in the past 21 years, and has shown a good trend where profit growth is superior to revenue growth. The life sciences business maintained stable profitability in 2023, and adjusted operating profit increased by about 19.4% to approximately US$78.3 million. Boom Biotech and Baisjie's adjusted operating profits in 2023 were approximately US$29.7 million in loss and US$2 million in profit, respectively. Legendary Biotech's adjusted operating loss narrowed to $395 million.
Furthermore, the company has sufficient cash reserves. In 2023, the company successively completed the financing of the three subsidiaries, Booming Bio, Legendary Biotech, and Baisjie, which will provide a solid guarantee for the capital expenses of the three subsidiaries in the next few years. As of December 31, 2023, the company had a cash reserve of approximately US$2 billion, including Legendary Biotech's US$1.3 billion, laying a steady foundation for the company's R&D and asset investment.
Chart 1: Company Revenue
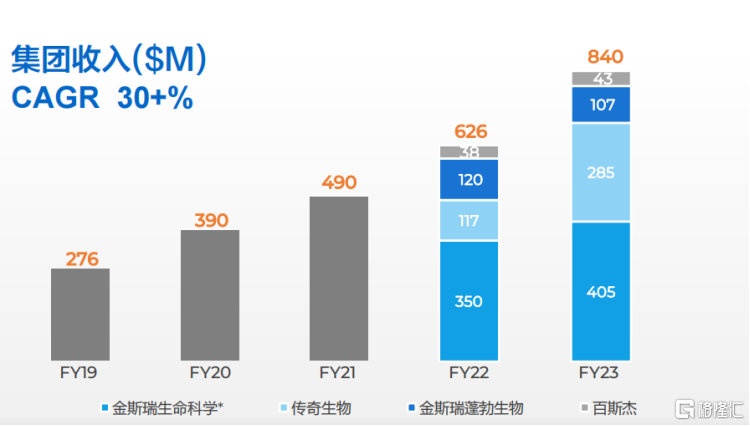
Data source: Company data, compiled by Gelonghui
All sectors develop steadily and build strong moats
1. Life Sciences Services and Products: Global Industry Leader, Significantly Increased Profitability
Judging from the market size, the life sciences market is very impressive. Whether it is antibody and protein engineering, gene and cell therapy industries, they are in a stage of rapid development. According to QYResearch, the global life science services market will grow from US$11.9 billion in 2021 to US$30 billion in 2028, with a compound growth rate of 14%.
The life sciences business is the cornerstone of Kingsley's business. It has had a very steady and excellent performance over a long period of time. According to the company announcement, despite changes in some external markets, the company's life science service and product business still showed a resilient growth trend in 2023. The profit growth rate was even higher than the revenue growth rate. After the adjustment, operating profit increased by about 19.4%, and profitability continued to increase.
Steady profit performance, thanks to the company's global strategic layout and close cooperation with top scientists in many industries, enables the company to more deeply grasp customer needs and increase customer stickiness. Recently, the company's Scientific Advisory Board (SAB) welcomed two very important new members: one is Dr. Carl June, a legend in the field of immunotherapy and director of the Parker Institute for Cancer Immunotherapy at the University of Pennsylvania; the other is Dr. David Liu, a leading scientist in the field of gene editing and a professor at Harvard University, Broad Institute, and Howard Hughes Medical Institute. I believe that the addition of two outstanding scientists will provide the company with deeper industry insight and promote the development of innovative services and products, thereby further empowering customers and achieving a wider range of commercial value. The addition of these two world-class scientists also shows their recognition of Kingsley's global development and international positioning.
Chart 2: The company accelerates breakthroughs in the life sciences sector
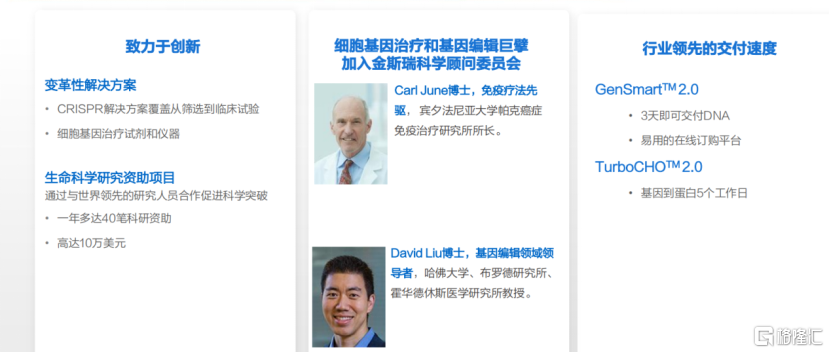
Data source: Company data, compiled by Gelonghui
2. Biopharmaceutical CDMO: Upgrading production capacity and increasing international competitiveness
As a new R&D and production outsourcing model in the pharmaceutical field, CDMO is temporarily under pressure in the short term. In the long run, with the gradual recovery of overseas investment and financing, the growth rate is expected to return to 11%/21% in 2024-25, and is expected to return further to 30%-40% after 2025.
Relying on technological advantages in the field of life sciences, Kingsley's subsidiary, Pengbo Biotech has a one-stop biopharmaceutical R&D and production platform, focusing on providing end-to-end CDMO services for cell and gene therapy (CGT) drugs, vaccines, and antibody protein drugs from target development to commercial production. It is worth noting that the company added its first 2000L CMO order, which became an important milestone in the CDMO business and an important step in the company's development into a global CDMO.
In terms of management teams, the company further enhances management team capabilities. In December 2023, Kingsley Boom Biotech appointed Dr. Chen Li as CEO. Dr. Chen Li has extensive experience in strategic planning and operation, and biological products business development. She has held important leadership positions in many well-known international pharmaceutical giants such as Sisi Pharmaceutical Services, Thermo Fei, and Merck. Dr. Chen Li's high sensitivity to emerging technologies and market opportunities has strongly promoted the company's business transformation, opened up new growth paths for the company, and enabled the company to maintain its leading position in a competitive industry.
In terms of capacity building, the company actively increases production capacity to meet market demand and expand the global production footprint. According to the company's plan, the first phase of commercial production capacity of 16,000L is expected in 2024. Among them, the GMP plasmid plant in the US is expected to be put into operation in the third quarter of 2024 and will provide competitive GMP plasmid services in the local market.
Figure 3: Global Production Planning

Data source: Company data, compiled by Gelonghui
3. Industrial synthetic biological products: far exceeding the industry's growth rate, rapid release of potential, marginal increase in profitability
Industrial enzyme preparations, also known as “biocatalysts”, are an important application of synthetic biology in the industrial field. They are mainly used in the fields of home care, food and beverage, biofuels, feed, agriculture, technology and medicine. According to Mordor Intelligence estimates, the global industrial enzyme market will reach US$11.02 billion in 2030, with a compound growth rate of 6.5% from 2020 to 2030.
Compared to the global competitive market for industrial enzyme preparations, the high-end Chinese market is difficult for ordinary players to enter due to the high entry threshold. As a representative of a local industrial enzyme company, Bestzyme (Bestzyme), a subsidiary of Kingsley, continues to expand its commercial share in the local market with its strong R&D and innovation capabilities, sufficient production capacity and mature commercial operation capabilities. With only single-digit growth in the market, Baisjie's core enzyme business revenue after deducting the exchange rate effect increased by 22.6%. In June 2023, Baisjie successfully obtained 250 million yuan in Series A financing. According to the company's forecast, after obtaining financing and carrying out related production capacity bottlenecks, Baisjie's production capacity is expected to increase by 30%-40%.
Judging from product progress, companies on the synthetic biology pipeline have continued to make progress and gradually move towards commercialization, showing strong commercial development potential. Among them, natural sweetener products have completed industrial-grade trial production, are planning to complete product safety verification, are preparing GRAS declarations, and are expected to be launched in 2024. Pilot production of lactoferrin products was completed in 2023. Currently, the company is conducting functional tests with partner universities and customers. The goal is to obtain GRAS certification in 2024 and begin industrial-grade trial production, which is expected to be launched on the market in 2025.
Figure 4: Synthetic Biology Pipeline

Data source: Company data, compiled by Gelonghui
4. Cell Therapy: A New Industry Benchmark, Accelerated Commercialization
As a model for independent innovative drugs going overseas, Legendary Biotech, a subsidiary of Kingsley, performed well in 2023.
In February 2022, Legendary Biotech's self-developed BCMA-targeted CAR-T cell therapy CILta-cel (Cilta-cel, trade name: Cilta-cel, trade name: Carvykti) was approved by the US FDA to treat patients with recurrent, difficult to treat multiple myeloma patients who have received at least four-line treatment. It became the world's second CAR-T therapy targeting BCMA, opening a new chapter for independent innovative drugs to participate in global market competition.
Judging from the sales situation, Legendary Biotech's commercialization capabilities are becoming a new benchmark in the CAR-T industry, and the market penetration rate is increasing at an accelerated pace. In 2023, the company's external revenue in the field of cell therapy was US$285 million, an increase of 144.2% over the previous year. Notably, Carvykti's sales performance in the first seven quarters after launch surpassed other CAR-T marketed products, treating more than 2,500 patients.
Judging from clinical data, compared to currently marketed drugs for multiple myeloma and other similar BCMA-targeted CAR-T products, the core product CILTA-CEL has significant advantages and has best-in-class potential. In 2023, Sidakiolens announced the patient report outcome (PRO) of the phase III study of CARTITUDE-4 at the ASH annual meeting, showing a good progression-free survival rate. The data showed that patients may have a higher health-related quality of life after a single injection of CARVYKTI.
In terms of capacity building, the company's overseas production capacity is rapidly expanding, which is expected to help sales continue to increase. In 2023, the company began clinical production at its production site in Belgium, and the cell processing capacity doubled compared to the beginning of 2023. In late December 2023, the company obtained approval to more than double the lentivirus production capacity from 20L to 50L. In addition, the company is also solving short-term production capacity shortages through cooperation with CMO. According to the plan, the company's CMO partner Novartis will start production of new plants in the US and Europe, and is expected to begin production of clinical materials in the first half of 2024. At the same time, Legend also signed a new production and supply service agreement with CMO partner Novartis for BCMA CAR-T products on March 27, 2024. The agreement expands the scope of cooperation between the two parties from clinical stage production to commercial supply, and is expected to continue to help the product's production capacity climb.
Chart 5: Carvykti's production capacity expansion

Data source: Company data, compiled by Gelonghui
It is worth noting that in addition to Sidakiolens, LEGENDARY BIOLOGY is also actively developing products such as CAR-T and CAR-NK for hematomas, solid tumors, and allogeneic CAR-NK. As testing progresses, other pipelines enter the harvest period in the future, and their value will gradually be discovered by the market.
Chart 6: Legendary Biologics Product Pipeline

Data source: Company data, compiled by Gelonghui
Summarize
Under the intertwined influence of the risk factors of globalization, how can enterprises persevere and continue to move forward in the midst of turbulence has become a common problem in the industry. However, Kingsley seemed to have given his own unique answer with an excellent questionnaire. One of the answers is “international” cooperation, which is more extensive global research and industry interaction, which has been deeply involved in life science business for more than 20 years. It is also a breakthrough to explore deeper commercial cooperation between legendary organisms and multinational pharmaceutical companies in the Xinjiang region of cell therapy. The second answer is “internal skill training”, which is a new path for biopharmaceutical CDMO to “further accelerate” through internal operation and management optimization, and a new stage of development after the start of financing for industrial synthetic biological products. As the company's steady growth trend shows, Kingsley's “internationalization” vision and “innovation power” drive the four major business segments, and I believe that corporate value will continue to steadily increase and be realized in the market.
