Gelonghui, April 8, 丨 Clover Bio-B (02197.HK) announced that in the phase I clinical trial of the bivalent RSV PreF-trimer subunit vaccine candidate SCB-1019 developed by the evaluation company based on Clover Biotech's unique Trimer-Tag (protein trimerization) vaccine technology platform, the first batch of young adults obtained positive preliminary immunogenicity and safety data.
In the phase I clinical trial, the first group of young adults (ages 18-59) received SCB-1019 or saline placebo, respectively, and the preliminary results of neutralizing the geometric mean titers (GMTs) and geometric multiples (gMFRs) of antibodies on day 0 (before vaccination) and day 28 (after vaccination) were as follows:
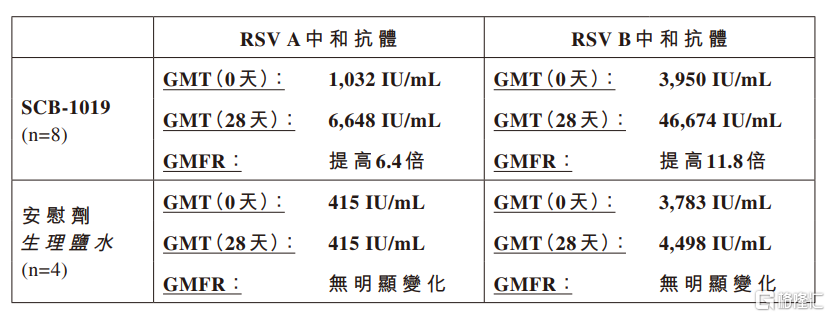
The RSV-A and RSV-B neutralization tests in this study were performed in a third-party testing laboratory using proven clinical testing methods and NIBSC16/284 reference standard serum. The test values were expressed in international units per milliliter (IU/ml).
Compared with RSVpref vaccines, the RSV vaccine candidate SCB-1019 developed by Clover Biotech is comparable or likely superior to initial immunogenicity data for RSV-A and RSV-B neutralizing antibodies. These positive immunogenicity data further support Clover Biotech's strategy of choosing to develop a bivalent RSV-A/BPrEF vaccine, because there have been data confirming that other monovalent vaccines targeting only RSV-A subtype have lower immune response and/or protective efficacy against RSV-B subtypes 1,4,5. This clinical result also confirmed that Clover Biotech's SCB-1019 PrEF antigen maintained a stable pre-fusion and trimer structure, and that exploratory immunogenicity results showed a neutralization site? This was further confirmed by a significant increase in competitive antibody titers. Furthermore, no obvious safety or reactivity issues were observed in the first batch of young adult subjects vaccinated with SCB-1019. Since then, the planned phase I clinical trial for the elderly group has progressed smoothly as scheduled.
The phase I clinical trial in Australia is a randomized, placebo-controlled study to evaluate the safety, reactogenicity, and immunogenicity of SCB1019 at different dosage forms and dosage levels in young adults and older adults. Safety and immunogenicity results for the elderly population are expected to be announced in the second half of 2024.
