“New quality productivity” is becoming one of the most popular terms recently.
In March 2024, the report on the work of the Chinese government identified “developing new quality productivity” as the top priority at present. Among them, innovative drugs are clearly mentioned for the first time in the “Actively Cultivating Emerging Industries and Future Industries” section, stating that it is necessary to “accelerate the development of the cutting-edge and emerging innovative drug industry, actively build a new growth engine for biomantry manufacturing, open up a new life science circuit, and create a number of pioneering zones for future industries” to add another spring breeze to the innovative drug circuit.
So, in an age where the innovative drug industry is receiving so much attention from top-level design, what investment opportunities are reflected behind it? What other businesses are worth checking out?
2024: Changes in the Age of Innovation
If you want to answer the first question, why don't you go firstTake a global perspective and explore changes in capital market capitalization.
Judging from the global market capitalization ranking, according to WIND data, pharmaceutical giants such as Eli Lilly (10th) and Novo Nordisk (12th) have appeared for the first time in nearly a year in the top 20 global market capitalization, marking a landmark rise in the global innovative pharmaceutical industry.
Judging from the global market capitalization ranking, according to WIND data, Nvidia, Broadcom, and Tesla have risen the fastest in the global market capitalization rankings in the past 10 years, according to WIND data. In the past 3 years, the fastest rising companies were the two leading innovative pharmaceutical companies, Eli Lilly and Novo Nordisk.
Overall,From the changes in the industry distribution and ranking of global market capitalization giants over the past 10 years, we can seePharmaceutical companies with real innovative power are crossing the industry cycle.In the future, the innovative drug industry chain combines the immediate needs of healthy consumption with the attributes of technological growth, and will become the standard for the capital market.
Looking back to the domestic market, China is a global innovation engine country, and investment opportunities for new quality productivity are being highlighted with policy encouragement.
The so-called new quality of productivity is characterized by innovation. The key is excellent quality, which is essentially advanced productivity. Judging from capital market performance, with policy support, the new productivity-related industrial chain is ushering in medium- to long-term investment opportunities.
According to WIND data, since 2018, the New Quality Productivity Index (8841755.WI) has fluctuated upward. Especially since 2023, the rise has been steep. It rose 44.94% for the full year of 2023, and obtained an excess yield of 49.79% compared to all A. Galaxy Securities predicts that with policy support, the new quality productivity-related industrial chain is expected to continue to grow in the future.
Among them, as an important component of new quality productivity, the innovative pharmaceutical industry is ushering in new opportunities for booming development under the two-wheel drive of policy technology.On the one hand, the government work report fully reflects the importance attached to the innovative drug industry. It is worth looking forward to favorable policies related to market pricing, medical insurance negotiations, and hospital admission to promote the development of China's innovative drug industry in 2024. On the other hand, technological advantages make Chinese innovative pharmaceutical companies internationally competitive in certain fields, and innovative pharmaceutical companies with real innovative capabilities are expected to stand out in the future.
Chart 1: New Quality Productivity Index Chart
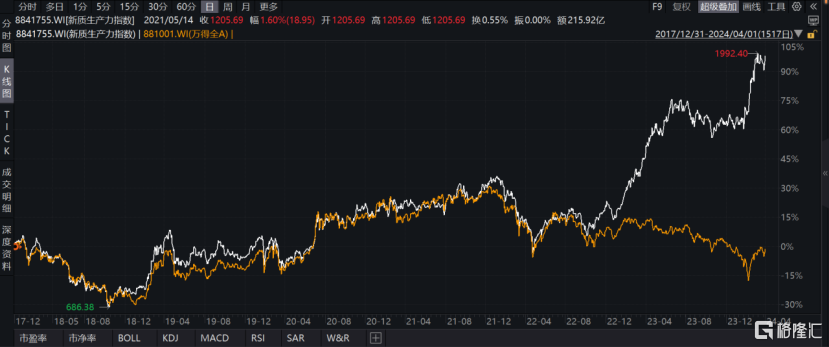
Data source: WIND, Gelonghui compiled data as of the closing date of April 1, 2024
White Line: New Quality Productivity Index Yellow Line: Wandequan A Index
Nuocheng Jianhua: A sample of high-quality development for innovative local pharmaceutical companies
It is undeniable that more and more high-quality samples are currently becoming the backbone of innovative local pharmaceutical companies, including Nuocheng Jianhua, which has been on the “Top 100 Innovative Chinese Pharmaceutical Companies” list for many years.
According to the announcement, Nuochengjianhua's R&D investment, which focuses on innovation at the source, increased 17.5% year-on-year in 2023 to reach 750 million yuan. Research projects continue to be enriched, global clinical progress continues to advance, making long-term preparations for more indications and new drug launches in the future, and continuously improving hematopoietic function.
Driven by continuous innovation, Nuochengjianhua's clinical value will continue to be transformed into commercial value, which can go through the cycle and become the internal driving force for the company's performance growth.
In terms of revenue, the company's revenue in 2023 rose 18.1% year on year to 740 million yuan. Among them, sales of the core product obutinib (Inochem) continued to grow, and revenue increased 18.5% year over year to reach 670 million yuan. In April 2023, a third new indication of obutinib was approved, and it successfully maintained the original price to renew the national health insurance contract. It became the first and only BTK inhibitor for MZL indications in China, effectively filling domestic gaps, accelerating the release of innovative value, and accessibility is constantly improving.
Furthermore, the CD19-targeted tamxitamab (tafasitamab) has been approved for marketing in Hong Kong and approved for use in Boao, Hainan and the Guangdong-Hong Kong-Macao Greater Bay Area, which will benefit patients with diffuse large B-cell lymphoma. Up to now, tamxitumab has been included in the overseas specialty drug catalogue of more than 27 provinces and cities in China, benefiting many patients. According to the company's plan, the registered clinical trial of tamxitumab combined with lenalidomide to treat relapsed/refractory DLBCL has completed patient enrollment in China. It is expected that BLA marketing applications will be submitted in the second quarter of 2024, and it is expected to be approved for marketing in the first half of 2025.
In the medium to long term, Nuochengjianhua is also actively exploring new potential targets. Many products are poised to launch, and performance is expected to continue to be realized.
Currently, the company has a total of 13 products under development that have entered the clinical stage, and the follow-up pipeline is rich. For example, in the BCL2 target field, which is currently popular in the market, Nuochengjianhua's ICP-248 stands out among many BCL2 inhibitors due to its excellent pharmacokinetic properties. The overall response rate (ORR) was 100% in patients who have been assessed, and the performance is promising. In January 2024, ICP-248 was approved by the US FDA to conduct clinical research. In March 2024, ICP-248 combined with obutinib for first-line treatment of CLL/SLL was clinically approved in China. In the long run, ICP-248 and obutinib have joined forces to form a strong combination effect, which is expected to become a key asset for Nuochengjianhua to achieve its globalization strategy. This strong combination of BTK+BCL2 will unleash huge market potential, and may become the highlight of the industry, leading the new trend in the innovative pharmaceutical industry.
In addition, Nuochengjianhua is also targeting the promising TYK2 market, thereby expanding the company's product layout in the field of self-defense.
Among them, the latest phase II data on ICP-332, a novel TYK2 inhibitor developed independently by the company to treat atopic dermatitis (AD), was released in the form of a late-breaking oral presentation (late-breaking oral presentation) at the 2024 American Academy of Dermatology Annual Meeting (2024 AAD Annual Meeting), showing excellent efficacy and safety, and reaching multiple efficacy endpoints. According to the plan, the company expects to launch a phase III clinical trial of ICP-332 for AD in China in 2024, clinical trials in the US, and a phase II trial for vitiligo as the second indication.
For markets in other self-immunity fields such as psoriasis, ICP-488, an oral highly selective TYK2 allosteric inhibitor independently developed by Nuochengjianhua, has completed phase I clinical studies and has shown good safety and tolerability. It is expected that clinical phase II patients will be enrolled in the first half of 2024.
Chart 2: Nuochengjianhua Pipeline Plan

Data source: Company data, compiled by Gelonghui
Summarize
CITIC Construction Investment pointed out, “From the perspective of optimizing resource allocation and institutional development, thoroughly implementing the national strategy, changes in the macroeconomic structure will lead the main direction of medium- to long-term investment.” It is undeniable that in an environment where there are still many uncertainties, focusing on the industrial chain related to new quality productivity will be one of the main logics for future long-term investors.
As a strategic emerging industry, the field of innovative pharmaceuticals is becoming a direction of increasing attention and cultivation at the national level, which will also present more investment opportunities. Among them, Nuochengjianhua, as a representative of innovative local pharmaceutical companies, can be described as a high-quality sample for high-quality development. In the 9th year of its establishment, Nuochengjianhua not only successfully listed on AH, successfully picked “-B”, commercialized its core products, but also accelerated its transformation to the better 2.0 version. The company not only has innovative strength at the source, has many global R&D product pipelines, but also has strong commercialization and production capabilities. However, this ability is more worthy of favor and attention in the face of industry fluctuations, and can be expected in the future.