At the National Conference this year, the government work report clearly identified “vigorously promoting the construction of a modern industrial system and accelerating the development of new quality productivity” as the top task of the government's work in 2024. Among them, the term “innovative drug” debuted and immediately sparked a buzz in the market.
From the previous “14th Five-Year Plan”, which listed the biomedical field as the top four key areas for priority development, to including innovative drugs as the focus of biomedical industry development in the government work report, it further highlights the importance that the country attaches to the development of the innovative pharmaceutical industry.
This will not only greatly stimulate the innovation vitality of pharmaceutical companies, but also indicate that the innovative pharmaceutical industry will enter a wider development space and become a new engine leading the growth of the industry.
With the current Hong Kong pharmaceutical companies successively releasing their 2023 results, the innovative capabilities and differentiation advantages of some pharmaceutical companies have gradually become apparent. To a certain extent, it also shows that the country's attention and support for the innovative pharmaceutical industry is being transformed into an actual driving force for enterprise development.
And Platinum Pharmaceuticals is one of them.
Financial reports show that in 2023, Hebo Pharmaceutical achieved revenue of 89.5 million US dollars, a year-on-year increase of 119.9%, and achieved its first annual profit. Net profit was 22.76 million US dollars, and profitability was quickly released.
On the expenditure side, the company has achieved remarkable results in operation and management. Expenses have dropped sharply. Annual R&D expenses were 45.08 million US dollars, down 66.6% year on year, and R&D efficiency continued to be optimized and improved; management expenses were 19.5 million US dollars, down 28.6% year on year, reflecting its refined management in terms of cost control.
Through this annual report, what is worth thinking about is what has been done right with Platinum Pharmaceuticals so that it can achieve annual profits? Can it provide some inspiration for the current development trend of innovative pharmaceutical companies?
Harbour Therapeutics pipeline progresses to break through again, creating new value for the market and patients
Heplatinum Pharmaceuticals' path to profit is no accident; behind it, there must be a series of wise strategic choices and efficient execution.
Since the end of 2022, Heplatinum Pharmaceuticals has officially divided its business into two major pillars: Harbour Therapeutics and Nona Biosciences (Nona Biosciences). The “Product Pipeline+Platform Cooperation” dual-driven business model not only gives full play to the company's advantages in product development and commercialization, but also achieves resource sharing and complementary advantages through close cooperation with external partners, and quickly promotes the company's profit and growth.
In terms of business distribution, on the one hand, Harbour Therapeutics continues to develop innovative global products with differentiated advantages through “independent development+global cooperation”, and has achieved breakthrough progress in many key treatment fields with accurate market positioning.
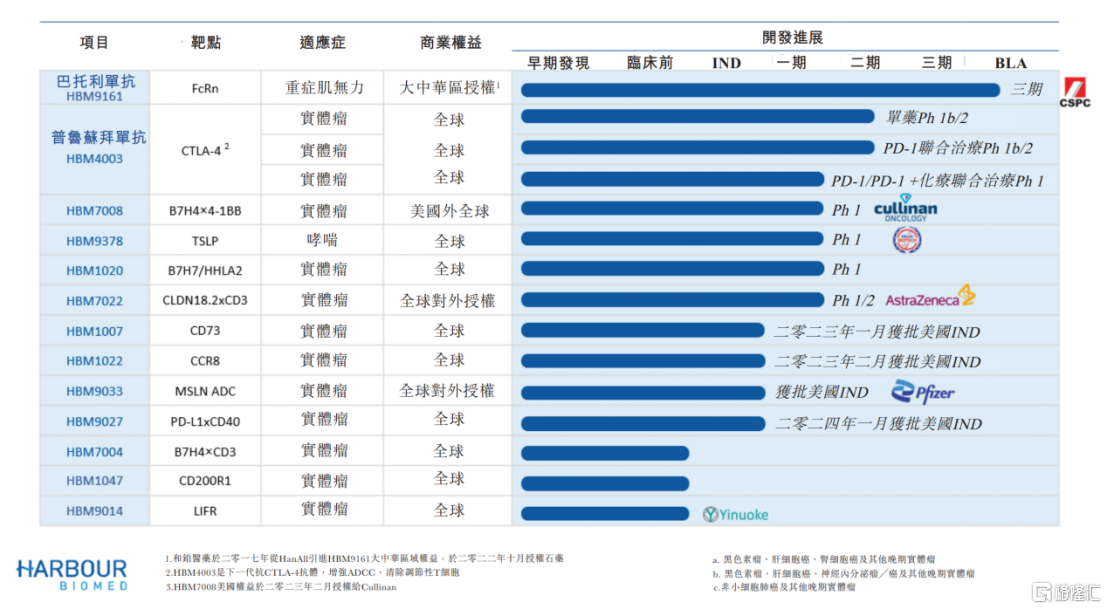
Currently, the company has more than 10 differentiated drug candidates, 4 of which are in the clinical stage. The main products include bartolizumab (HBM9161), prusubimab (HBM4003), HBM7008, and HBM1020.
Source: Financial Report
It is worth noting that one of the key criteria for evaluating the innovative value of a product is whether it can bring value to the market and patients.
The author believes that Heplatinum Pharmaceuticals has achieved this. Simply look at it from the following three aspects:
First, the company's product pipeline has the potential to be applied to multiple indications, as well as first-of-its-kind and best-in-class, which is expected to fill the gaps in the current market treatment field.
For example, as the innovative drug with the fastest clinical progress, HBM9161 is not only the first anti-FcRN therapy with complete data in Greater China, but also showed good efficacy and safety in the phase III trial of systemic myasthenia gravis.
HBM4003 is an anti-CTLA-4 all-human monoclonal heavy chain antibody developed based on the Harbour Mice HCaB platform. Many global clinical trials, including melanoma, neuroendocrine tumors, and hepatocellular carcinoma, have shown strong efficacy and safety, and have the potential to become the next generation of tumor immune cornerstone therapy.
Furthermore, relying on the development technology of the company's Harbour Mice H2L2 transgenic mouse platform, HBM1020 became the world's first B7H7/HHLA2 monoclonal antibody officially approved to enter the clinical phase. Currently, it has been administered to the first patient in a phase I clinical trial in the US.
Second, the successful licensing of a number of innovative products not only enabled the company's pipeline products to enter the global market faster and increase market coverage, but also demonstrated the leadership of the company's technology platform, as well as its uniqueness and forward-looking nature in selecting drug development targets.
In February 2023, the company first signed an exclusive license for HBM7008 with Cullinan Oncology Inc. in the US. It will receive an advance payment of 25 million US dollars, a milestone payment of up to 600 million US dollars, and a high double-digit percentage of tiered license fees. Immediately after that, a global development and commercialization licensing agreement for HBM7022 was reached with AstraZeneca in April, and phase I/II clinical trials for solid tumors are currently being initiated globally.
Third, the company's technological innovation fields cover cutting-edge technology fields such as dual/multiple antibodies, ADC, mRNA, and Car-T. Compared with traditional treatments such as small-molecule drugs and monoclonal antibodies, these drugs have shown higher efficacy and safety, and have huge commercial value potential.
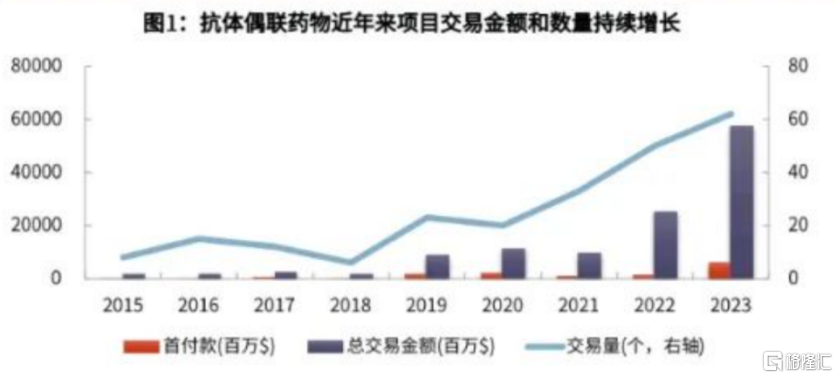
Taking ADC as an example, ADC drugs combine monoclonal antibodies with potent cytotoxic drugs, and have both the sense of direction of targeted drugs and the strong effects of chemotherapy. They are known as “magic bullets” in the field of anti-tumor treatment, and continue to attract well-known domestic and foreign pharmaceutical companies to step up. In terms of market size, Frost & Sullivan predicts that the global ADC market is expected to reach 63.8 billion US dollars in 2030, and the domestic ADC market is expected to reach 68.9 billion yuan in 2030, which indicates that ADC drugs will play an increasingly important role in the field of anti-tumor treatment in the future.
Data source: Everbright Securities
Deepening the global network, Nona Biotech continues to inject vitality into global biopharmaceutical innovation
On the other hand, Nona Biotech's core technology platform continues to empower global biopharmaceutical innovation. It has not only achieved remarkable achievements in technological innovation, but also successfully transformed many scientific research results into actual productivity, bringing considerable economic benefits to the company.
Simply put, based on a powerful antibody discovery platform, it has spawned various application platforms including protein engineering platforms, ADC development platforms, and GPCR drug development platforms, and continues to advance its “antibody+” strategy through platform innovation, licensing and services to provide comprehensive antibody solutions for global partners.

Source: Company Information
Up to now, Nona Biotech has accumulated more than 50 partners from 0 to now, and is promoting more than 30 cooperative projects, and more than 19 of these projects have successfully entered the clinical development stage. This not only shows the appeal of the company's technology platform, but also highlights its deep application and influence in the field of next-generation innovative biopharmaceuticals.
According to the author's incomplete statistics, looking at the cooperation trends in 2023 alone, Nona Biotech has reached strategic cooperation with 12 world-renowned pharmaceutical companies and institutions, covering a wide range of innovative drug fields such as ADC, dual antibodies, and novel proteolytic therapeutics.

Source: Company Information
Among them, the exclusive global clinical development and commercialization licensing agreement between Nona Biotech and Pfizer for HBM9033, an ADC drug targeting human mesothelin (MSLN), attracted particular attention from the industry.
Under the agreement, Nona Biotech will receive an advance payment of 53 million US dollars and recent payments, and a milestone payment of up to 1.05 billion US dollars. In addition, Nona Biotech will also be eligible for tiered royalties ranging from high single digits to high ten digits from net sales.
As an innovative drug developed by Nona Biotech through its proprietary platform Harbour Mice, HBM9033 has the potential to be the world's best-in-class treatment. This collaboration with Pfizer undoubtedly marks an important milestone in the development of the Harbour Mice platform and ADC ecosystem. This has not only effectively strengthened Nona Biotech's global cooperation network, but also further expanded the scientific and commercial value of the company's technology platform, laying a solid foundation for future innovative drug development and market competition.
It is easy to see that with the continuous release of the value of the Nona Biotech platform, it is gradually becoming an important engine for the company's performance growth, injecting strong impetus into the company's continuous development.
The market is showing positive changes, and innovative pharmaceutical companies may welcome a new round of growth
Despite many concerns in the market, the current performance of innovative pharmaceutical companies in the capital market is somewhat unsatisfactory. However, it is undeniable that the market is showing some positive changes and signals, providing favorable conditions for the recovery of the innovative pharmaceutical industry, and innovative pharmaceutical companies such as Platinum Pharmaceuticals are also ushering in rare opportunities.
For example, a “Full Chain Implementation Plan to Support the Development of Innovative Drugs (Draft for Comments)” has recently been circulating in the market, all of which comprehensively promote key aspects of R&D, approval, use, and payment of innovative drugs. At the same time, as expectations of US interest rate cuts and expectations of continued interest rate cuts in the domestic market increase, the market has shown signs of a reversal at the bottom.
Major brokerage agencies and senior analysts are also optimistic about the future trend of the innovative drug sector. For example, Yang Song, chief analyst of the pharmaceutical industry at Tianfeng Securities, said that he is optimistic about investment opportunities in the entire pharmaceutical sector in 2024; the Southwest Securities Research Report points out that under overseas macro-interest rate cuts, the three dimensions of policy, valuation, and fundamentals are superimposed, and the pharmaceutical industry still has structural opportunities, and innovation+going overseas is still the main optimistic idea.
Based on this, the author believes that the current overall valuation of Heplatinum Pharmaceuticals is in a historically low range, and the downward momentum is limited. Under the combined effects of positive changes in industry policy and other factors, as Heplatinum Pharmaceuticals officially enters the profit stage, mutual collaboration between the two core business pillars will bring stable cash flow to the company. These funds will continue to feed back the upgrading of the company's technology platform and R&D of innovation pipelines, thus forming a virtuous cycle, stimulating its long-term growth potential, and ultimately fully reflected in the performance of the capital market.
