Source: Wall Street News
The CXO sector has once again been hit hard.
Pharmacology Biotech held a business exchange meeting before the market today to lower revenue expectations for some business segments. Among them, it is expected that in 2023, pharmaceutical development (D) revenue will drop by 18%-20%, and production (M) revenue will drop by 15%-18%. Due to two revenue declines, the full-year revenue growth forecast was lowered from 30% to around 10%. As a result of the two businesses, drug development (D) and production (M) together account for about 70% of revenue, and since fixed assets are allocated according to 40% growth, profits directly dragged down will turn negative this year.
As a result, Yakuming Biotech's stock price dived sharply at the opening, and trading was temporarily suspended during the intraday period. At the same time, it also led to a sharp adjustment in the stock price of the entire CXO sector.
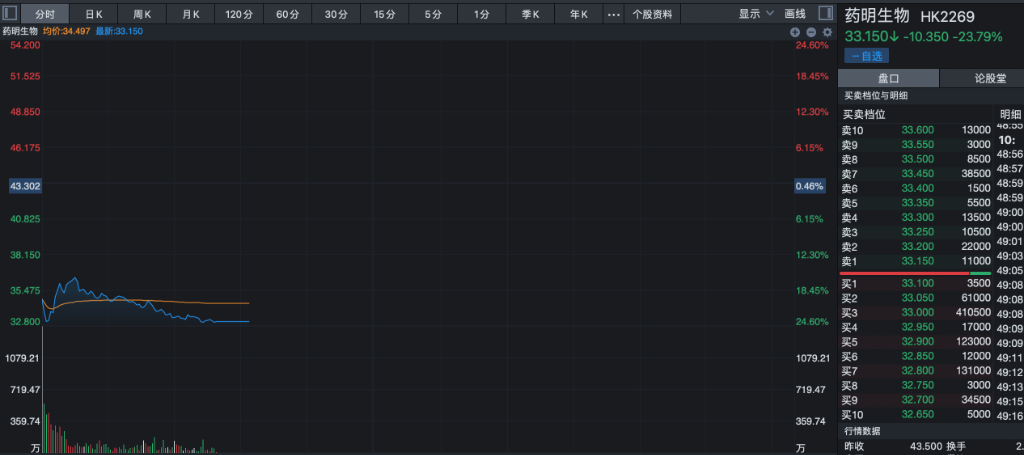
In the current downturn in performance expectations, although the market already anticipated a decline in new drug development orders due to the decline in industry investment and financing conditions, the market did not fully anticipate the delay in large orders in the production business and the decline in profits due to overseas operations.
But most importantly, the company chose to adjust its full-year operating expectations in December, especially after its subsidiary Pharmaceuticals Joint Stock Exchange went public, which raised market doubts about the company's transparency and communication strategy.
Which businesses have fallen short of expectations? Where is the market's question about Pharmacovigilance?
The current 23-year guidance reduction mainly comes from 3 parts:
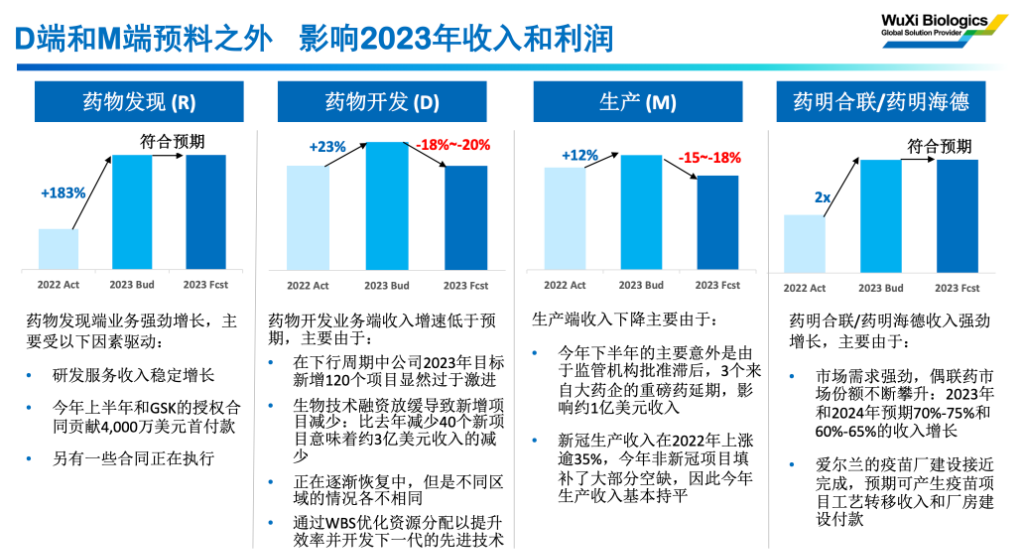
1) New drug development (D) is less affected by industry investment and financing than expected, and is expected to affect revenue of 300 million US dollars.
The CEO of Pharmaceutical Biology said at the communication meeting that the new order signed fell short of expectations, so this year's new drug development (D) business revenue will be reduced by 300 million US dollars from expectations.
According to the development schedule of pharmacology biology, the New Drug Development (D) business segment only signed new orders in the first half of the year was able to contribute to performance in that year. Even though orders were restored in the second half of the year, these orders did not contribute substantially to the year's results.

Pharmaceutical Biotech set a goal of 120 new molecules throughout the year, but completed only 46 new orders in the first half of the year, which fell far short of expectations. Although order growth in the second half of the year resumed, as of the end of the third quarter, there were only 61 participants. By November 30, the total number of new orders signed had reached only 91, far from reaching the target. Obviously, the company's original goals were too aggressive, and the situation was clear before the end of the 3rd quarter.
This situation means:
The adjustment of performance expectations should have occurred earlier: Pharmaceutical Biotech's downward guidance on Phase D business was supposed to be carried out at least at the end of the third quarter, but the company delayed this adjustment, which may cause the market to question the company's transparency and timeliness.
Market expectations of a decline in Phase D business: Although the market expects a decline in Pharmacology's Phase D business, the company's handling of this information may have affected investors' confidence in its future performance predictions.
2) The extension of three major drugs from major pharmaceutical companies affected revenue of about 100 million US dollars.
The CEO of Yao Ming Biotech said that the main accident in the second half of the year was due to delays in regulatory approval. Regarding the production (M) part, delays in major customer products were not recent, and the company could also choose to communicate with the market in advance. This decline in performance exceeded market expectations, but this delay is not something that can only be anticipated in the near future.
3) On the profit side, the commencement of operations at overseas factories caused gross profit losses of about 100 million US dollars.
Due to the fact that overseas factories began operating, profits were affected in the short term, resulting in a decline in gross profit of about 100 million US dollars in '23. This also exceeded market expectations.
Of course, investors' concerns about geopolitical influence cannot be falsified for some time to come, even though the company says it will maintain this leading edge in manufacturing quality compared to competitors such as Samsung Biotech.
Most importantly, however, Pharmacology Biotech's timing for lowering its full-year performance guidelines in early December raised doubts in the market, especially after Pharmacovigilance was just launched. The market doubts that the announcement could have been issued earlier, but the company chose to make adjustments after the spin-off and listing. This timing choice may cause the market to question the company's motives and trigger a more intense market reaction.
However, as a brother company, Yao Ming Kangde's performance was also affected by the industry's investment and financing boom, but the company announced its three-quarter report. The market did not react very much at the time, and there was little discussion.
Earlier, in the three-quarter report exchange, Yao Ming Kangde mentioned, “Since demand in the early drug development stage in the fourth quarter fell short of expectations, some related laboratory business revenue is expected to fall short of initial expectations. Therefore, the company adjusted the revenue growth range in 2023 from 5-7% to 2-3%; excluding COVID-19 commercialization projects, it was adjusted to 25-26% from the previous 29-32%.”
The biopharmaceutical CDMO still has growth opportunities, but structural changes will become the main theme in 2024
However, in communication, Pharmaceutical Biotech still has full confidence in the second half of 2024. The CMO (production) business will continue to grow well in the second half of next year. On the one hand, due to the delay of this year's three major projects, and on the other hand, the progress of projects within the pipeline itself.
At the same time, the company believes that Pharmacology Biotech's revenue and profit growth will still return to 30% growth in 2025.
However, 2024 may indeed usher in a glimmer of light for the biopharmaceutical CDMO.
According to three venture capital firms interviewed by Endpoints News, as early biopharma funding stabilizes, biotech companies may abandon in-house manufacturing to save cash, and clinical-stage CDMO demand is expected to increase by 2024.
At the same time, at this stage, biotech companies that do not need to invest in production facilities will be more favored by capital. And although pharma's funding will be stable, it will still be limited, which means that biopharmaceutical companies may continue to reduce their divisions. This could include shrinking in-house manufacturing departments, leading to more outsourcing opportunities.
In summary, Pharmaceutical Biotech's overconfident performance guidelines in 2023 to final misplaced results have once again proved that during the overall downturn in the industry, it is difficult for even excellent companies to fully withstand the impact of the industry cycle.
For the company, communicating honestly with the market and updating performance expectations in a timely manner is the key to winning the trust of investors.
Editor/Jeffrey
