Compared to other Red Sea markets, this RDN circuit, which is still in the Blue Ocean market, not only has global medical device giants betting, but also many excellent domestic local companies struggling to catch up.
Hypertension, as a common cardiovascular disease, has a total of 1.4 billion hypertensive patients worldwide (data source: WHO), with a huge base. Although medication is currently the main treatment for high blood pressure, long-term blood pressure control through lifestyle intervention and medication is less than 14% worldwide, and the current situation of hypertension treatment is in urgent need of improvement.
Thankfully, as scientists have gone through decades of innovative research, renal artery desympathetic neurosurgery (RDN) has emerged, which will become a disruptive treatment method leading minimally invasive surgery to treat high blood pressure. Recently, good news has also been spreading frequently.
On November 17, Medtronic (Medtronic) announced that its 14-year Symplicity SpyralTM renal artery sympathetic nerve multipolar radiofrequency ablation catheter system has been approved for marketing by the US Food and Drug Administration (FDA) for the treatment of high blood pressure.
Not long ago, Recor Medical and its parent company, Otuka Medical, announced that the FDA has approved Recor's Paradise ultrasound renal deneurological system, which is suitable for adjuvant treatment of hypertensive patients with lifestyle changes and where antihypertensive drugs cannot fully control blood pressure, bringing more antihypertensive options for hypertensive patients.
At the same time, good news also came from domestic and local medical device companies. On November 18, at the latest 14th Annual Meeting of the Cardiovascular Committee of the Cross-Strait Hospital Health Exchange Association and the Strait Cardiovascular Disease Health Development Academic Conference, Professor Jiang Xiongjing's team, deputy director of the Vascular Center of Fuwai Hospital of the Chinese Academy of Medical Sciences, and Professor Ye Tao's team successfully completed a case of RDN surgery using the Iberis multi-electrode renal artery radiofrequency ablation catheter system from the domestic manufacturer Baixin'an. The patient is a 33-year-old female who was found to have a systolic pressure of 200 mmHg when donating blood ten years ago. Currently, I am taking 5 types of antihypertensive drugs, and my systolic pressure is still not up to standard. The systolic pressure in the blood pressure clinic on the second day after surgery has dropped below 120 mmHg, and the drug is currently being discontinued for further follow-up.
Chart 1: Demonstration of RDN surgery
Data source: 14th Annual Meeting of the Cardiovascular Committee of the Cross-Strait Hospital Health Exchange Association and the Straits Cardiovascular Disease Health Development Academic Conference, compiled by Gelonghui
The Baixinan Iberis multi-electrode renal artery radiofrequency ablation catheter system launched post-marketing clinical radius-HTN in Europe in May 2022. The main researcher is Professor Felix Mahfoud, a professor from Saarland University Hospital, Homburg/Saar, who is also the chairman of the Arterial Hypertension Working Group of the German Society of Cardiology. RADIUS-HTN is designed as a 1:1 comparison between radial artery access and femoral artery, highlighting the advantages of Baixinan Iberis as the only RDN product compatible with radial artery access in the world. There are fewer complications with radial artery access, and the advantages of day surgery to reduce overall treatment costs have been recognized by European doctors. Currently, a total of 15 cases have been enrolled in Europe.
Industry insiders pointed out that RDN treatment is expected to become the “third carriage” for the treatment of high blood pressure. So, how effective is this new treatment? What step have players in this field taken now?
Medtronic takes the lead, and domestic brands are catching up
Since RDN is a type of minimally invasive surgery, it destroys the renal artery nerve through minimally invasive surgery, and has a lifelong antihypertensive effect of 7*24 hours per operation. This procedure has many advantages such as few side effects, high safety, reduced patient medication cycle, and improved prognosis for hypertensive patients. Today, RDN therapy has received consensus recommendations from many experts, and has become a competitive betting direction for many players in the cardiovascular field.
Take global medical device giant Medtronic as an example. After developing better multi-electrode second-generation RDN products, Medtronic continued to work hard to prepare for the subsequent commercialization of Symplicity Spyral. According to information, Medtronic is already actively carrying out relevant preparations for commercial insurance access and sales team training in the US. According to forecasts from investment banks such as Morgan Stanley and Bank of America and Medtronic, RDN products will become one of Medtronic's next major growth points. Even if calculated based on a 1% penetration rate, it will be able to reach a market size of more than 3 billion US dollars.
At the same time, domestic manufacturers are also catching up in the RDN field, and are even catching up head-on. Among them, Baixinan, Xinmai, and Meiliweiye have all completed clinical trials.
However, compared to RDN products currently being developed at home and abroad, Baixin'an's core product, Iberis 2nd, has a clear competitive advantage. As the only RDN product with multiple electrodes in the world that also allows intervention through the femoral artery and through the radial artery, it has now obtained CE certification to enter the European market, and has also entered the green channel for approval of NMPA innovative medical devices domestically.
Judging from the product design, Baixinan's Iberis 2nd has an innovative and differentiated design. The main features include: (1) the ring-shaped ablation catheter tip, which provides stronger radial support to ensure fit to the blood vessels; (2) the outer diameter of the 4F ablation catheter, compatible with the 6F catheter sheath, to achieve full coverage of the main renal artery and its branches; (3) it can fully adapt to the different openings of the renal artery; (4) multi-electrode design (4 electrodes).
Among these features, the multi-electrode design can increase ablation efficiency (total treatment time for each kidney is less than 20 minutes) and reduce the time doctors and patients are exposed to radiation during surgery. Furthermore, the multi-electrode design makes the ablation position more uniform, reducing the impact of a single electrode on the final surgical outcome due to the doctor's reliance on experience and feel.
In addition, Iberis 2nd also allows doctors to choose between transfemoral intervention and transradial intervention (currently most RDN is femoral artery intervention, radial artery access is less traumatic, can achieve day surgery, less harm to patients, and can reduce overall treatment cycle and cost), which provides doctors with more flexible options and can determine more suitable solutions based on specific conditions. For patients, RDN surgery through radial artery access is more minimally invasive, which means fewer complications and faster recovery after surgery.
Judging from the R&D progress, in January 2022, Baixinan completed the enrollment of all Iberis 2nd domestic patients, making it the first domestic manufacturer to complete clinical enrollment. Since there are no commercialized RDN products in China, it can be said that Baixinan has strong competitiveness and a first-mover advantage in both domestic and foreign markets. According to the forecast of Huatai Securities, China's RDN market is expected to reach 10.5 billion yuan by 2030.
Chart 2: RDN's competitive pattern and market space in the Chinese market
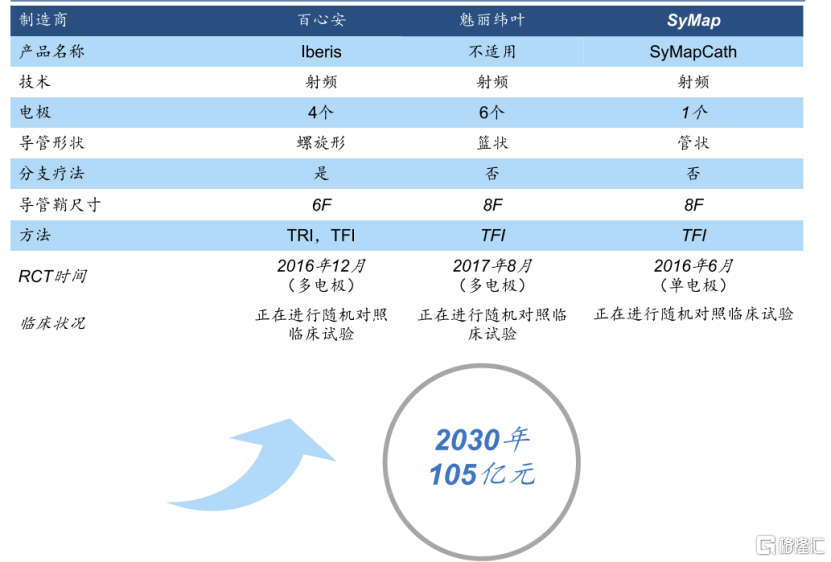
Data source: Frost & Sullivan, Huatai Securities, compiled by Gelonghui
RDN: A rising star in the field of hypertension treatment
Looking back at the development history of RDN treatment, although there have been many twists and turns, recent developments have provided strong evidence for RDN treatment of hypertension. Globally, including the US, Europe, and Asia, expert consensus has been issued, and the approval of Medtronic and Recor's RDN products by the FDA further indicates a wave of promotion of this technique around the world.
As early as 1923, some doctors proposed the theory of using neurosurgery alone to treat high blood pressure. In 2009, the renal artery sympathetic ablation catheter was born, kicking off the prelude to minimally invasive renal sympathetic ablation treatment for high blood pressure. Many medical device giants have placed bets one after another.
Subsequently, Medication (later acquired by Medtronic for 800 million US dollars) released two multi-center clinical trials, HTN-1 and HTN-2, proving that RDN is a safe and effective treatment for patients with intractable hypertension and can achieve long-lasting lowering of blood pressure. However, in 2014, Medtronic announced the HTN-3 experiment. Due to the experimental design, which did not meet expectations, global RDN research fell into a period of confusion, and market sentiment about RDN cooled down.
Although Medtronic's original product failed, today's review is more due to the limitations of clinical trial design and imperfect surgical methods at the time. As the clinical trial of Symplicity SpyralTM, Medtronic's next-generation RDN product, received positive results, the importance of bifurcation ablation was emphasized in clinical practice, and market attention was rekindled after being certified as a “breakthrough therapy” by the FDA.
Thankfully, in recent years, clinical data performance in the RDN field has been spreading good news. Excellent clinical data also confirms that RDN is becoming the brightest star in hypertension treatment methods.
In 2021, the Symplicity SpyralTM catheter system completed the first clinical case in Boao, Hainan; at the 2022 TCT conference, Medtronic revealed that patients treated with RDN in the past still showed significant drops in blood pressure over a long period of time; at the 2022 AHA conference, Medtronic disclosed the final clinical data of its RDN product Symplicity Spyral; in November 2023, Medtronic's Symplicity Spyral finally knocked on the FDA's approval and was successfully listed in the US. Up to now, more than 25,000 patients have been treated with Medtronic Symplicity series radiofrequency ablation systems worldwide.
Chart 3: Medtronic RDN Test History

Data source: public information, compiled by Gelonghui
Judging from the market size, the RDN market is on the trillion-dollar gold circuit. It is the single most revolutionary product in the medical device field, none of which is one of the most revolutionary products in the medical device field.
According to Frost & Sullivan data, global annual medical expenses for the treatment of high blood pressure were about 400 billion US dollars in 2019, and medical expenses are expected to continue to increase in the future. However, in the Chinese market, there were more than 300 million hypertensive patients in 2019. Of these, only 23.5% had blood pressure well controlled, the number of patients with uncontrolled hypertension reached 200 million, and the number of patients with intractable hypertension reached 47.61 million.
Judging from the clinical data performance, the clinical results of Iberis HTN announced by Baixinan at the 2023 CIT Conference (China Interventional Cardiology Conference) are also particularly eye-catching.
Iberis HTN (NCT02901704) is a prospective, multicenter, blind, randomized controlled trial led by Academician Gao Runlin and Professor Jiang Xiongjing of Fuwai Hospital. It aims to evaluate the safety and efficacy of the Iberis multi-electrode renal artery radiofrequency ablation catheter system in treating primary hypertension. A total of 217 participants were enrolled.
On the one hand, the clinical results of Iberis HTN showed excellent efficacy. According to the results, the renal artery ablation (RDN) group reached the main clinical endpoint of efficacy (change in mean systolic pressure compared to baseline during dynamic blood pressure for 6 months and 24 hours after surgery) and was significantly superior to the sham surgery group. Compared to baseline, the RDN group reduced the 24-hour systolic pressure ABPM by 11.93 mmHg and the sham surgery group by 2.58 mmHg. The difference in pressure reduction between the RDN group and the control group was 9.35 mmHg and was statistically significant (P<0.0001).
On the other hand, clinical results of Iberis HTN showed safety. According to the results, no serious adverse events associated with the device were observed. The effectiveness and safety of Iberis has been verified in trials.
Chart 4:24-hour changes in blood pressure 6 months after random grouping compared to baseline

Data source: 2023CIT Conference, compiled by Gelonghui
Summarize
According to public statistics, Medtronic has invested more than 2 billion US dollars in the RDN project, and the total investment of major global manufacturers in RDN has also exceeded 5 billion US dollars. The reason why the industry is optimistic about the RDN field comes, on the one hand, from the broad market space for chronic diseases, and on the other hand, from the clinical data that is frequently reported.
Compared to other Red Sea markets, this RDN circuit, which is still in the Blue Ocean market, not only has global medical device giants betting, but also many excellent domestic local companies struggling to catch up. Among them, Baixin'an, which has differentiated innovative designs on a global scale, has higher barriers to competition and is worthy of attention and anticipation.
