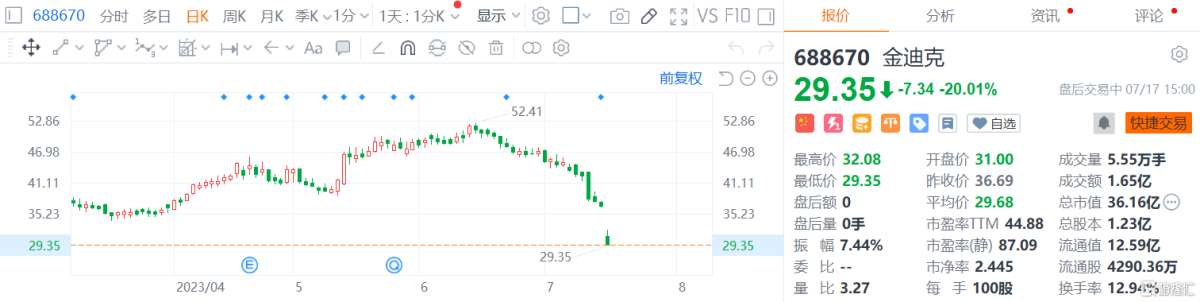
This morning (July 17), A-share vaccine company Kim Dick opened sharply lower20cmStopping,It recorded 7 consecutive market declines, with a cumulative decline of 34.72% in the past 7 days. CurrentlyThe report hit a six-and-a-half-month low of 29.35 yuan, with a total market capitalization of 3.61 billion yuan.
According to the news, recently, heavy rain occurred in the Taizhou Pharmaceutical High-tech Zone (Gaogang District) in Jiangsu Province, where the company is located. Affected by this, the company's plant was flooded. The company decided to temporarily stop production of the influenza vaccine workshop from July 15, 2023.

The plant was flooded, and Q3 revenue is likely to be 0
According to data, the company belongs to the pharmaceutical manufacturing industry and is a biopharmaceutical enterprise focusing on R&D, production and sales of vaccines for human use. The quadrivalent influenza virus lysis vaccine developed by the company was marketed and sold in November 2019. It is the only quadrivalent influenza virus lytic vaccine in China that has been declared to CDE as a class 1 biological product for preventive use.
According to the announcement, currentlyAll operating income comes from the quadrivalent influenza vaccineInfluenza vaccine production and sales have obvious seasonal characteristics. Every year from September to December is the peak season for influenza vaccine sales.This temporary suspension of production will cause the product to go on sale later than in previous years. It is expected that it will be more difficult to market and sell in September when the 2023-2024 influenza season officially starts, which will adversely affect the company's business performance. It is currently impossible to determine the exact time for the company to resume production, and the specific losses cannot be calculated for the time being.
Regarding the current shutdown of the influenza vaccine workshop,Kim Dick expects the company's revenue to be 0 from July to September 2023The year-on-year decline was significant, and the impact on annual sales performance cannot be assessed yet.
In addition to the impact of the temporary shutdown of production caused by the torrential rain, Jindick also said that in 2023, the company obtained the strain and standard products for the 2023-2024 influenza season relatively late, and production progress lagged behind previous years.
Performance declined for two consecutive years
In terms of performance, the annual report data for the past two years showed that Kim Dick's performance continued to decline.
In 2021, Jindick's revenue was 392 million yuan, down 33.41% from the same period in previous years; net profit was 82.46 million yuan, down 46.79%. In 2022, Jindick's revenue and net profit both fell again, down 18.81% and 49.62% year-on-year respectively.

Regarding the decline in performance, the company said that the factors affecting the company's business performance came from various aspects: reduced personnel turnover, difficulties with terminal vaccination; expansion of business scale, and progress of projects under construction and R&D projects.
However, due to the high incidence of influenza in many parts of the country in the first quarter of this year, Golden Dick's performance in the first quarter of this year ushered in a major explosion, with revenue exceeding 100 million yuan, an increase of 751.94% over the previous year.
What is the impact of the vaccine market in the second half of the year?
According to information,Currently, there are 7 quadrivalent influenza vaccines approved for marketing in China. The main manufacturers include Hualan Biotech, Jindik, Changchun Institute, Wuhan Institute, Kexing Biology, Shanghai Institute, Guoguang Biology, and Sanofi.
Among them, Jindik achieved 1,3496 million doses and 4.243,300 doses of the quadrivalent vaccine in 2019 and 2020, respectively, accounting for 13.9% and 12.63% of the total number of quadrivalent vaccine batches, ranking second and third among domestic quadrivalent influenza vaccine manufacturers, respectively.
From Yangcheng Evening News,The influenza vaccines produced by vaccine companies this year have been put on the market one after another.
On July 6, China Biology announced that China's first universal quadrivalent influenza virus lysis vaccine for people over 6 months of age, produced by Sinopharm Group China Biology's Shanghai Institute of Biological Products, has been approved and certified and is newly marketed.
Prior to that, on June 29, Hualan Vaccine and Beijing Kexing Biological Products Co., Ltd. both announced the launch of their quadrivalent influenza vaccine. Among them, the Hualan vaccine said that in 2023, the first batch of quadrivalent influenza virus lysis vaccines in the country was shipped from Xinxiang, Henan. With the current influenza vaccine delivery, this year's influenza vaccination work has also begun in full.Kexing also said that the quadrivalent influenza virus lytic vaccine produced has been issued in batches by the China Food and Drug Testing Research Institute.
Also, in terms of fund holdings, the data shows that as of the first quarter of 2023, 2 public funds held heavily in Kim Dick. Among them, the public fund holding the largest number was Red Earth Innovation Enhancing Yield Bond A.