TENX: Second Quarter Results
TENX: Second Quarter Results
By John Vandermosten, CFA
約翰·範德莫斯滕(John Vandermosten),CFA
NASDAQ:TENX
納斯達克:TENX
READ THE FULL TENX RESEARCH REPORT
閲讀完整的TENX研究報告
Second Quarter 2021 Financial and Operational Review
2021年第二季度財務和運營回顧
Tenax Therapeutics, Inc. (NASDAQ:TENX) reported second quarter 2021 results via the issuance of a press release and the filing of Form 10-Q to the SEC on August 16, 2021.
Tenax治療公司(納斯達克市場代碼:TENX)通過發佈新聞稿並於2021年8月16日向證券交易委員會提交10-Q表格,公佈了2021年第二季度業績。
Highlights for the second quarter and to-date:
第二季度和到目前為止的亮點:
➢ Publication of HELP trial results - April 2021
➢發佈HELP試驗結果-2021年4月
➢ Addition of company Russell Microcap Index - June 2021
➢新增公司羅素微市值指數-2021年6月
➢ Announced CEO transition - July 2021
➢宣佈首席執行官交接-2021年7月
➢ $10 million PIPE ATM offering - July 2021
➢1,000萬美元管道自動取款機服務-2021年7月
➢ KOL webinar on Levosimendan in PH-HFpEF - August 2021
2021年8月在PH-HFpEF舉行的關於左西孟丹的➢kol網絡研討會
➢ Publication highlighting novel levosimendan MOA in PH-HFpEF - August 2021
➢在PH-HFpEF-2021年8月發佈重點介紹新的左西孟丹MOA
Tenax produced no revenues during 2Q:21 and incurred operating expenses of $1.96 million resulting in net loss of ($1.72) million, or ($0.10) per share.
Tenax在2Q:21期間沒有產生收入,發生了196萬美元的運營費用,導致淨虧損(172萬美元),或每股虧損(0.10美元)。
For the quarter ending June 30, 2021 versus the quarter ending June 30, 2020:
截至2021年6月30日的季度與截至2020年6月30日的季度相比:
➢ General and administrative expenses increased 46% to $1.27 million from $869,000 driven by reimbursement of approximately $358,000 in legal fees associated with arbitration proceedings in the prior year as well as increases in Board of Directors fees and capital market expenses, partially offset by a reduction in investor relations services behind the difference;
➢的一般和行政費用從上一年的869,000美元增加到127萬美元,這主要是由於上一年與仲裁程序相關的法律費用報銷約358,000美元,以及董事會費用和資本市場費用的增加,但差額背後的投資者關係服務減少部分抵消了這一增長。
➢ Research and development expenses decreased 46% to $693,222 from $1,274,837 with the conclusion of Phase II HELP and associated CRO and patient enrollment costs, partially offset by imatinib formulation development contributing to the change;
隨着第二階段HELP以及相關的CRO和患者登記費用的結束,➢的研究和開發費用從1,274,837美元下降到693,222美元,下降了46%,部分被促成這一變化的伊馬替尼配方開發所抵消;
➢ Other income increased to income of $247,820 from a loss of ($2,101) with forgiveness of Paycheck Protection Program (PPP) loan;
➢的其他收入從損失(2,101美元)增加到247,820美元,免除了Paycheck Protection Program(PPP)貸款;
➢ Net loss was ($1.72) million versus ($2.14) million, or ($0.10) and ($0.23) per share, respectively.
➢淨虧損為(172萬美元),而不是(214萬美元),或每股虧損(0.1美元)和(0.23美元)。
At the end of the second quarter, cash, equivalents and marketable securities totaled $2.18 million, compared to $6.71 million at the end of 2020. Following forgiveness of the PPP Tenax holds no debt on its balance sheet. Cash burn for the six months ended June 30th totaled $5.07 million. Following the end of the quarter, Tenax raised gross proceeds of $10 million from a securities purchase agreement with an institutional investor. Management has guided that cash reserves, including the $10 million recently secured, are sufficient to sustain the firm through 2Q:22.
截至第二季度末,現金、等價物和有價證券總額為218萬美元,而2020年底為671萬美元。在購買力平價獲得豁免後,Tenax的資產負債表上沒有債務。截至6月30日的六個月的現金消耗總計507萬美元。季度結束後,Tenax通過與一家機構投資者達成的證券購買協議籌集了1000萬美元的毛收入。管理層指導説,現金儲備,包括最近獲得的1000萬美元,足以維持公司到2Q:22。
KOL Webinar on Levosimendan for PH-HFpEF
關於左西孟丹用於PH-HFpEF的Kol網絡研討會
Tenax announced, on August 5th, a Key Opinion Leader (KOL) webinar that was held on August 16, 2021 at 10 AM ET. The event focused on the current treatment landscape and therapeutic potential of levosimendan in pulmonary hypertension with heart failure with preserved ejection fraction (PH-HFpEF). The webinar featured KOL Daniel Burkhoff, MD, Ph.D., member of Tenax' Scientific Advisory Board, who discussed the unmet medical need in treating patients with PH-HFpEF and how Tenax' levosimendan could become a new treatment option. Stuart Rich, M.D., Tenax' Chief Medical Officer then discussed clinical development plans for levosimendan.
Tenax於8月5日宣佈,關鍵意見領袖(KOL)網絡研討會於2021年8月16日美國東部時間上午10點舉行。這次活動的重點是左西孟丹在肺動脈高壓伴保留射血分數的心力衰竭(PH-HFpEF)中的治療前景和治療潛力。Tenax公司的科學顧問委員會成員、醫學博士KOL Daniel Burkhoff參加了這次網絡研討會,他討論了在治療PH-HFpEF患者方面未得到滿足的醫療需求,以及Tenax公司的左西孟丹如何成為一種新的治療選擇。Tenax的首席醫療官、醫學博士斯圖爾特·裏奇隨後討論了左西孟丹的臨牀開發計劃。
Dr. Burkhoff began with an overview of HFpEF, exercise hemodynamics, stressed blood volume and its role in exercise hemodynamics comparing between normal and HFpEF patients, and the effects of levosimendan in this context. Preserved ejection fraction includes patients with 50% ejection fraction and greater, which can include a variety of underlying pathophysiology. Symptoms for these patients include congestion, enlarged left atria, and exercise intolerance. Pulmonary hypertension is common in those with HFpEF, and for those with PH-HFpEF, prognosis is worse. Patients are diagnosed using an assessment of hemodynamic exercise test, namely supine bicycle while catheterized to measure a panel of pressure metrics. Measurement of patients during sustained exercise allows segmentation of the severity of PH-HFpEF. Those with PH-HFpEF show a distinct, dynamic increase in pulmonary wedge pressure under exercise. Central venous pressure is also increased, allowing insight into the underlying physiology. Some mechanistic theories have suggested the ventricle as a driver in the condition, including decrease in chamber size, abnormal stiffness, and other aspects directly related to the ventricles. More recently, potential tests shifted to peripheral factors such as the inability of arteries to vasodilate, decreased oxygen extraction from the muscle and venoconstriction. Venoconstriction represents a novel mechanism relating to levosimendan's efficacy that had not been significantly studied due to technological limitations.
伯克霍夫博士首先概述了HFpEF、運動血流動力學、應激血容量及其在運動血流動力學中的作用,比較了正常和HFpEF患者之間的差異,以及左西孟丹在這方面的作用。保留的射血分數包括射血分數在50%或更高的患者,這可能包括各種潛在的病理生理因素。這些患者的症狀包括充血、左心房增大和運動不耐受。肺動脈高壓在HFpEF患者中很常見,PH-HFpEF患者預後較差。患者是通過血流動力學運動試驗的評估來診斷的,即在插管時仰卧自行車測量一組壓力指標。在持續運動期間測量患者可以分割PH-HFpEF的嚴重程度。PH-HFpEF患者在運動時表現出明顯的動態肺楔壓升高。中心靜脈壓也會升高,從而可以深入瞭解潛在的生理機制。一些機械理論認為,在這種情況下,腦室是驅動因素,包括腦室大小減小,異常僵硬,以及其他與腦室直接相關的方面。最近,潛在的測試轉向了外周因素,如動脈無法擴張血管,肌肉氧攝取量減少和靜脈收縮。靜脈收縮代表了一種與左西孟丹療效相關的新機制,由於技術限制,尚未對其進行重大研究。
In particular, volume distribution and volume metabolism in the body may both be modulated with sympathetic activation. The acute effects of exercise, which is one form of sympathetic activation, results in rapid mobilization of venous reservoir, causing venoconstriction leading to increase in effective circulating blood volume, or stressed blood volume. The sympathetic nervous system and its interaction with venous system create conditions where relatively small changes in venous property can result in potent regulation of stressed blood volume. In PH-HFpEF, patients' resting stressed blood volume is increased compared to normal, and they have a greater increase in stressed blood volume compared to normals during exercise. Among the many factors that change during exercise, heart rate, contractility and systemic vascular resistance are primarily responsible for increase in cardiac output and blood pressure observed during exercise. Keeping all factors constant, changing only venous compliance, changes in stressed blood volume account for almost all changes in central venous pressure (CVP) and wedge pressure, suggesting stressed blood volume as a therapeutic target. Tenax' HELP trial, after six weeks, saw almost no change in placebo group in CVP or wedge pressure at rest or with legs up versus the treated group that had 5 mm reduction in CVP and wedge pressure at rest and with legs up. Analysis of stressed blood volume found no significant change in the placebo group whereas a 500 mL reduction in stressed blood volume was observed in the treated group. This was in line with expectations as results from a previous study1 (Fudim et al.) at Duke University which showed that splanchnic nerve block, which is responsible for venoconstriction, reduced resting and exercise-induced pulmonary arterial and wedge pressure.
具體地説,體內的容量分佈和容量代謝都可能受到交感神經激活的調節。運動是交感神經激活的一種形式,運動的急性效應會導致靜脈儲存庫的快速動員,導致靜脈收縮,導致有效循環血量增加,或應激性血量增加。交感神經系統及其與靜脈系統的相互作用創造了條件,在這種條件下,靜脈特性的相對較小的變化可以導致對應激血容量的有效調節。PH-HFpEF組患者的靜息應激血容量較正常增加,運動時應激血容量增加幅度較大。在運動過程中改變的眾多因素中,心率、收縮力和全身血管阻力是運動過程中觀察到的心輸出量和血壓增加的主要原因。保持所有因素不變,僅改變靜脈順應性,應激血容量的變化幾乎可以解釋中心靜脈壓(CVP)和楔壓的所有變化,這表明應激血容量是治療的靶點。Tenax‘Help試驗在6周後發現,與治療組相比,安慰劑組靜息或抬腿時的中心靜脈壓(CVP)和楔壓幾乎沒有變化,而治療組休息時和抬腿時的中心靜脈壓和楔壓降低了5 mm。應激血容量分析發現,安慰劑組沒有明顯變化,而治療組則觀察到應激血容量減少了500mL。這與之前一項研究的結果一致。1(福田等人。)杜克大學(Duke University)的一項研究表明,負責靜脈收縮的內臟神經阻滯可以降低靜息和運動誘導的肺動脈和楔壓。
Dr. Stuart Rich recapitulated many of the points that Dr. Burkhoff made. Rich emphasized that HFpEF, which is group 2 of pulmonary hypertension, is considered the largest unmet medical need in cardiovascular medicine at this time, and that PH-HFpEF is a distinct pathology. Every approved pulmonary vasodilator has been tried in PH-HFpEF and failed. Levosimendan is not only a calcium channel activator, as it was developed as an inotrope to treat systolic heart failure, but it is also a potent potassium channel activator of ATP type. And this was shown when levosimendan-treated patients displayed improvements in pressure metrics that translated to statistically significant exercise tolerance improvement, an important feature in securing market approval. Tenax is now in the process of transitioning intravenously-administered patients to an oral formulation that were in the open label extension. This process is expected to be complete by 4Q:21. We expect to see a 2022 start to a Phase III trial for levosimendan.
斯圖爾特·裏奇博士重述了伯克霍夫博士提出的許多觀點。裏奇強調,HFpEF是肺動脈高壓的第二組,被認為是目前心血管醫學中最大的未得到滿足的醫療需求,PH-HFpEF是一種獨特的病理。所有批准的肺血管擴張劑都在PH-HFpEF中嘗試過,但都失敗了。左西孟丹不僅是一種鈣通道激活劑,因為它被開發為一種肌力抑制劑,用於治療收縮性心力衰竭,而且它也是一種有效的ATP類型的鉀通道激活劑。當接受左西孟丹治療的患者表現出壓力指標的改善,轉化為統計上顯著的運動耐量改善時,這一點就得到了證明,這是獲得市場批准的一個重要特徵。Tenax目前正在將靜脈給藥的患者過渡到開放標籤擴展中的口服制劑。這一過程預計將在4Q:21之前完成。我們預計將於2022年開始左西孟丹的第三階段試驗。
Novel Levosimendan MOA in PH-HFpEF
PH-HFpEF中新型左西孟丹MOA
On August 12th, Tenax issued a press release announcing the publication of a new study identifying a novel mechanism of action behind the improved cardiovascular hemodynamics and exercise tolerance observed in Phase II HELP study. The publication, entitled "Changes in Stressed Blood Volume with Levosimendan in Pulmonary Hypertension from Heart Failure with Preserved Ejection Fraction: Insights Regarding Mechanism of Action," was published in the Journal of Cardiac Failure. Through an in-depth analysis of data from the HELP Study, the authors elucidated the underlying mechanism and found that the reductions in pulmonary wedge pressure (PCWP) and central venous pressure (CVP) did not depend on any effect of the drug on the force or speed of contraction of cardiac muscle. Instead, the study concluded that the reduction in PCWP and CVP was attributable to levosimendan's effect on K+ATP channel activation, lowering stressed blood volume (SBV), or the volume of blood that is being pushed by the heart. The work validated that dilating the splanchnic2 circulation will lower SBV and by extension CVP and PCWP in PH-HFpEF as observed in the HELP trial.
8月12日,Tenax發佈了一份新聞稿,宣佈發表一項新的研究,確定了在第二階段HELP研究中觀察到的心血管血流動力學和運動耐量改善背後的一種新的作用機制。這篇題為“左西孟丹對射血分數保留的心力衰竭所致肺動脈高壓的應激性血容量的變化:對作用機制的見解”的文章發表在心力衰竭雜誌。通過對HELP研究數據的深入分析,作者闡明瞭其潛在的機制,發現肺楔壓(PCWP)和中心靜脈壓(CVP)的降低並不依賴於藥物對心肌收縮力或收縮速度的任何影響。相反,這項研究得出的結論是,PCWP和CVP的降低歸因於左西孟丹對K+ATP通道激活的影響,降低了應激血容量(SBV),即心臟推動的血量。這項工作證實了擴張內臟2循環可以降低PH-HFpEF的SBV,進而降低中心靜脈壓和PCWP,正如HELP試驗中所觀察到的那樣。
DiTonno Retires, Giordano Takes the Helm as CEO
迪託諾辭職,佐丹諾掌舵擔任首席執行官
Announced on July 7th, Tenax announced that CEO Anthony DiTonno retired, effective July 13, 2021. DiTonno served as CEO, since 2018, and Director for Tenax. The Board has appointed Christopher Giordano to serve as Tenax' CEO, effective July 14, 2021. To facilitate the transition, Giordano will serve as an employee of Tenax starting July 6 until he begins his role as CEO.
7月7日宣佈,Tenax宣佈首席執行官安東尼·迪託諾(Anthony DiTonno)退休,從2021年7月13日起生效。DiTonno自2018年以來一直擔任Tenax的首席執行官和董事。董事會已任命克里斯托弗·佐丹諾(Christopher Giordano)擔任Tenax公司的首席執行官,從2021年7月14日起生效。為了促進過渡,佐丹諾將從7月6日開始擔任Tenax的員工,直到他開始擔任首席執行官。
$10 Million PIPE ATM Offer
1000萬美元的管道自動取款機優惠
Tenax disclosed that it had entered into a definitive agreement with a single healthcare-focused institutional investor for issuance and sale of 4,773,269 units at $2.095 per unit, with each unit consisting of one unregistered, pre-funded warrant to purchase one share of common stock and one unregistered warrant to purchase one share of common stock for a total of 9,546,538 shares underlying the warrants. The unregistered pre-funded warrants are immediately exercisable, have an exercise price of $1.97 per share and will expire 5.5 years from date of issuance.
Tenax披露,它已與一家專注於醫療保健的機構投資者達成最終協議,以每單位2.095美元的價格發行和出售4773269股,每個單位包括一個購買一股普通股的未登記預籌資權證和一股購買一股普通股的未登記認股權證,總共9546,538股認股權證。未登記的預籌資權證可立即行使,行權價為每股1.97美元,自發行之日起將到期5.5年。
HELP Trial Results
幫助試驗結果
In June 2020, Tenax announced topline results from its Phase II Hemodynamic Evaluation of Levosimendan in Patients with PH-HFpEF (HELP). Results were positive and statistically significant for key measures critical to a successful Phase III trial. HELP is the first study conducted in PH-HFpEF patients to show material positive improvements in hemodynamics and 6-minute walk distance. The distance for the six minute walk test,3 which has been used as a primary endpoint in other pulmonary hypertension trials, was 29 meters greater for the levosimendan group compared to the placebo group. This measure had a p-value of 0.0329, better than the 0.05 threshold required for statistical significance. We discussed the details of this trial in a previous report.
2020年6月,Tenax宣佈了其第二階段的背線結果H情緒動力學E估值:LEosimendan inP使用PH-HFpEF的患者(幫助)。對於對成功的第三階段試驗至關重要的關鍵指標,結果是積極的,在統計學上具有重要意義。HELP是第一個在PH-HFpEF患者中進行的研究,顯示出血流動力學和6分鐘步行距離的實質性積極改善。六分鐘步行測試的距離,3在其他肺動脈高壓試驗中被用作主要終點,左西孟丹組比安慰劑組多29米。這一指標的p值為0.0329,好於統計學意義所需的0.05%的臨界值。我們在之前的一份報告中討論了這次試驗的細節。
The Phase II trial provided substantial support for advancing levosimendan to the next stage that includes publication of results in a major cardiovascular journal, request for an end-of-Phase-II meeting with the FDA and preparation for a Phase III trial.
第二階段試驗為將左西孟丹推進到下一階段提供了實質性支持,包括在一家主要心血管雜誌上發表結果,要求與FDA舉行第二階段結束會議,以及為第三階段試驗做準備。
End-of-Phase II Meeting with FDA
與FDA的第二階段會議結束
In October 2020, Tenax met with the FDA for an end-of-phase II meeting to discuss Phase II HELP trial data. The FDA agreed that one or two Phase III trials, depending on size, with primary endpoint of change in 6-minute walk distance over 12 weeks or a single Phase III trial with clinical worsening over 24 weeks would be sufficient to demonstrate efficacy. The FDA has indicated that a composite primary endpoint, which will include the six minute walk test, will be required. The agency also highlighted the necessity of a safety database, which should be addressed as Phase III protocol is finalized and submitted.
2020年10月,Tenax與FDA舉行了第二階段結束會議,討論第二階段HELP試驗數據。FDA同意,根據規模的不同,以12周內6分鐘步行距離變化為主要終點的一項或兩項III期試驗,或臨牀惡化超過24周的單一III期試驗,將足以證明療效。FDA已經表示,將需要一個複合主要終點,其中將包括6分鐘步行測試。該機構還強調了安全數據庫的必要性,該數據庫應在第三階段協議最終敲定並提交時加以解決。
Next Steps
接下來的步驟
Tenax is currently switching from infused to oral levosimendan in patients who participated in the open-label extension of the HELP study, who continue treatment and remain eligible to participate in the HELP study amendment. The next regulatory contact will likely be prior to year end. The additional meeting with the FDA is necessary to report on results related to the shift to oral from infused levosimendan. In parallel with the crossover work with the oral dosage, Tenax is identifying sites for the upcoming Phase III. Institutional review boards (IRBs) are granting approvals at the sites and preparation for registrational studies is advancing as expected. Management has guided toward a 2022 start to the Phase III trial.
Tenax目前正在將參與HELP研究開放擴展的患者從輸液改為口服左西孟丹,這些患者繼續接受治療,並仍有資格參加HELP研究修正案。下一次監管接觸可能會在年底之前。與FDA的額外會議是必要的,以報告與輸注的左西孟丹轉變為口服有關的結果。在口服劑量交叉工作的同時,Tenax正在為即將到來的第三階段確定地點。機構審查委員會(IRBs)正在批准這些地點,註冊研究的準備工作正在按預期進行。管理層已指導將於2022年開始第三階段試驗。
While it is too early to determine Phase III trial design, based on commentary we anticipate a composite primary endpoint that will include the six minute walk test. Our best estimate is that continued preparatory work will take place in 2021 and first patients will be enrolled in 2022. A rough estimate of time, cost and size of the registrational trial range from 18 to 36 months, $30 to $50 million and 200 to 300 patients. While these estimates have not been confirmed by the company, we believe they are reasonable based on precedent.
雖然現在確定第三階段試驗設計還為時過早,但根據評論,我們預計將有一個包括6分鐘步行測試的複合型主要終點。我們的最佳估計是,2021年將繼續進行準備工作,2022年將招收首批患者。註冊試驗的時間、成本和規模粗略估計為18至36個月,3,000萬至5,000萬美元,200至300名患者。雖然這些估計沒有得到該公司的證實,但我們認為,根據先例,這些估計是合理的。
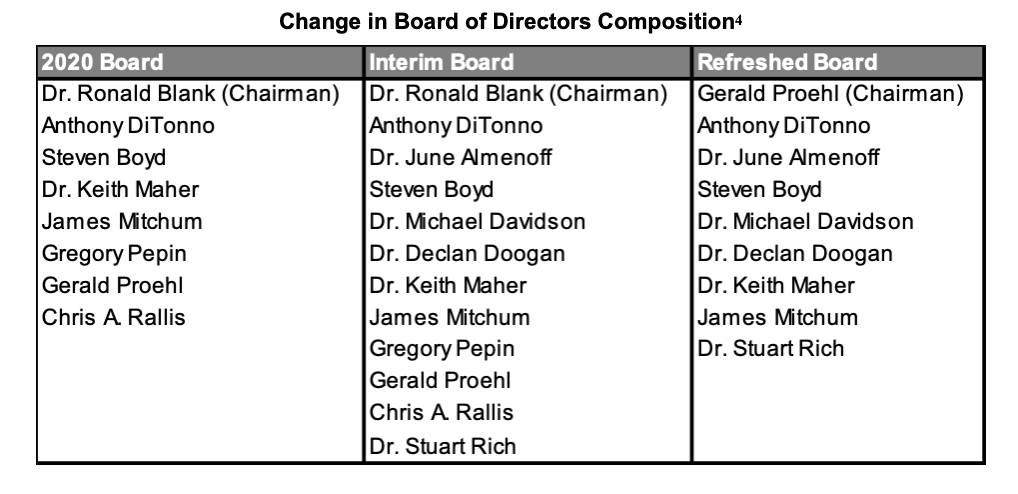
Additions to the Board
管理局的新增成員
Tenax added four new members to its board of directors, which was announced on March 2nd, 2021. The new directors included June Almenoff, MD, Ph.D., Michael Davidson, MD, Delcan Doogan, MD, and Stuart Rich, MD, who was recently appointed the company's Chief Medical Officer.
Tenax在3月2日宣佈董事會增加了四名新成員發送,2021年。新董事包括醫學博士瓊·阿爾門諾夫、醫學博士邁克爾·戴維森、醫學博士德爾坎·杜根和醫學博士斯圖爾特·裏奇。裏奇最近被任命為該公司的首席醫療官。
Dr. Almenoff brings over 20 years of senior leadership experience in the biopharma space. She served as President and Chief Medical Officer of Furiex Pharmaceuticals, which was acquired by Actavis (now AbbVie), and held various ascending positions at GlaxoSmithKline for 12 years. Dr. Almenoff is currently Chief Scientific Officer of RedHill Biopharma (NASDAQ:RDHL) and also serves on the investment advisory board of Harrington Discovery Institute and on the boards of Brainstorm Cell Therapeutics (NASDAQ:BCLI) and Kurome Therapeutics. Dr. Almenoff received her bachelor's from Smith College and graduated with AOA honors from the MD-Ph.D. program at Mount Sinai School of Medicine, completing post-graduate medical training at Stanford University Medical Center.
阿梅諾夫博士在生物製藥領域擁有20多年的高級領導經驗。她曾擔任被Actavis(現為AbbVie)收購的Furiex製藥公司的總裁兼首席醫療官,並在葛蘭素史克(GlaxoSmithKline)擔任了12年的各種晉升職位。阿爾梅諾夫博士目前是Redhill Biophma公司(納斯達克市場代碼:RDHL)的首席科學官,也是哈靈頓發現研究所的投資顧問委員會成員,以及頭腦風暴細胞治療公司(納斯達克市場代碼:BCLI)和黑石治療公司的董事會成員。Almenoff博士從史密斯學院獲得學士學位,並以AOA榮譽畢業於西奈山醫學院的MD-Ph.D.項目,並在斯坦福大學醫學中心完成研究生醫學培訓。
Dr. Michael Davidson was founder and former Chief Scientific Officer of Corvidia Therapeutics, which was acquired by Novo Nordisk. He is Clinical Professor and Director of the Lipid Clinic at the University of Chicago Pritzker School of Medicine. Dr. Davidson co-founder and Chief Medical Officer of Omthera Pharmaceuticals, acquired by AstraZeneca Pharmaceutical. He also founded the Chicago Center for Clinical Research that was acquired by Pharmaceutical Product Development. Dr. Davidson received his bachelor's and master's from Northwestern University and MD from Ohio State University School of Medicine.
邁克爾·戴維森博士是Corvidia治療公司的創始人和前首席科學官,該公司被諾和諾德公司收購。他是芝加哥大學普利茲克醫學院(University Of Chicago Pritzker School Of Medicine)的臨牀教授和血脂診所主任。戴維森博士是Omthera製藥公司的聯合創始人兼首席醫療官,該公司被阿斯利康製藥公司收購。他還創立了芝加哥臨牀研究中心,該中心被製藥產品開發公司收購。戴維森博士在西北大學獲得學士和碩士學位,在俄亥俄州立大學醫學院獲得醫學博士學位。
Dr. Declan Doogan adds over 30 years of pharma and biotech industry experience. He served as Senior Vice President and Head of Worldwide Development at Pfizer and held positions at Pfizer in the US, UK and Japan. Dr. Doogan also held positions as CMO and acting CEO of Amarin (NASDAQ:AMRN) and is Chairman and co-founder of Biohaven (NYSE:BHVN). He serves on a number of Board appointments and received his MD from Glasgow University.
德克蘭·杜根博士擁有30多年的製藥和生物技術行業經驗。他曾擔任輝瑞公司高級副總裁兼全球發展主管,並在輝瑞公司美國、英國和日本擔任過職務。杜根博士還擔任過Amarin公司(納斯達克市場代碼:AMRN)的首席營銷官和代理首席執行官,是Bioaven公司(紐約證券交易所市場代碼:BHVN)的董事長和聯合創始人。他在多個董事會任職,並獲得格拉斯哥大學的醫學博士學位。
On April 7, three long-serving directors elected to step down. Ronald R. Blanck, D.O., Gregory Pepin and Chris A. Rallis will depart their posts on the Board of Directors of Tenax Therapeutics, effective as of the Annual Meeting of Stockholders scheduled for June 10, 2021.
4月7日,三名長期任職的董事選擇辭職。羅納德·R·布蘭克(D.O.)、格雷戈裏·佩平(Gregory Pepin)和克里斯·A·拉利斯(Chris A.Rallis)將辭去他們在Tenax治療公司董事會的職務,從定於2021年6月10日召開的年度股東大會起生效。

PH Precision Med Acquisition
PH Precision Med收購
On January 19, 2021, Tenax announced the acquisition, through merger with wholly owned subsidiary Life Newco II, of privately held, clinical stage PH Precision Med (PHPM). The transaction was completed on January 15, 2021, in an equity deal valued at approximately $21.6 million, which we discuss here. PHPM's shareholders were issued the equivalent of 12.124 million equity shares as consideration for the deal, comprised of 1,892,905 shares of Tenax common stock, representing ~15% of Tenax' shares outstanding, and 10,232 shares of Class B Preferred Stock, which convert to 10,232,000 shares of common stock, pending stockholder approval. Tenax is required to hold a stockholder meeting no later than July 31, 2021 to obtain stockholder approval. If not approved, meetings will be held every 90 days to seek approval until either approved or the convertible preferred stock is no longer outstanding. Based on the closing price of Tenax on January 18, 2021 of $1.78, the acquisition is valued at approximately $21.6 million.
2021年1月19日,Tenax宣佈通過與全資子公司Life Newco II合併,收購私人持股的臨牀期PH Precision Med(PHPM)。這筆交易於2021年1月15日完成,股權交易價值約2160萬美元,我們在這裏討論。PHPM的股東獲得了相當於1212.4萬股股本的交易對價,其中包括1,892,905股Tenax普通股,約佔Tenax已發行股票的15%,以及10,232股B類優先股,這些優先股將轉換為10,232,000股普通股,等待股東批准。Tenax被要求在2021年7月31日之前召開股東大會,以獲得股東批准。如果不獲批准,將每90天召開一次會議尋求批准,直到獲得批准或可轉換優先股不再流通股。根據Tenax在2021年1月18日的收盤價1.78美元計算,此次收購的估值約為2160萬美元。
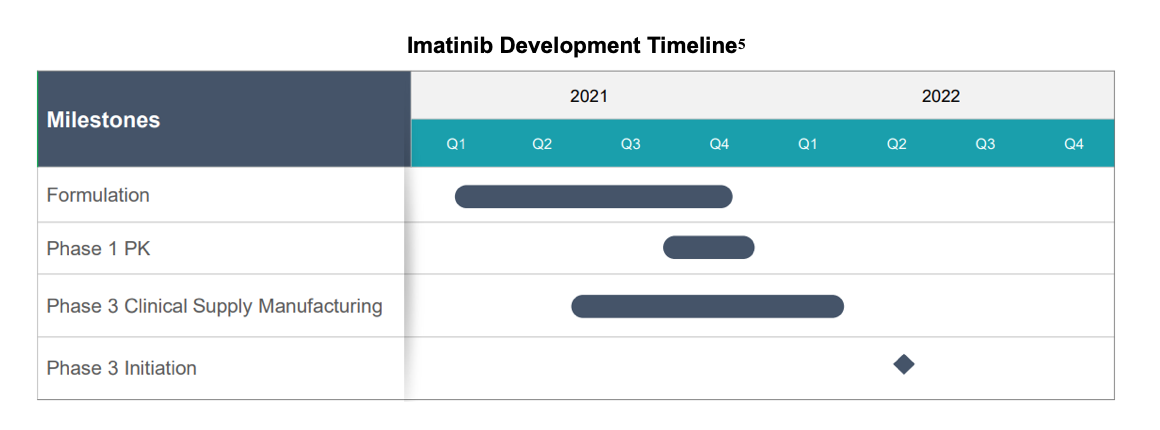
To add detail to Tenax' recent acquisition of PH Precision Med and its candidate imatinib, Tenax hosted a web conference on January 21, 2021, a few days after the acquisition was announced. We discuss the specifics of the deal, provide background on imatinib and its use in PAH and review existing therapies for the indication in a previous report. Efforts are now underway to identify a formulation of the drug using an enteric coating to address GI-related side effects that were identified in previous trials. A pharmacokinetic (PK) study testing the new formulation is expected to be conducted and provide results in 2H:21.
為了補充Tenax最近收購PH Precision Med及其候選公司伊馬替尼的細節,Tenax在宣佈收購幾天後,於2021年1月21日舉辦了一次網絡會議。我們討論了交易的細節,提供了伊馬替尼及其在PAH中使用的背景,並在之前的一份報告中審查了現有的適應症治療方法。目前正在努力確定一種使用腸溶包衣的藥物配方,以解決先前試驗中確定的與胃腸道相關的副作用。測試這種新配方的藥代動力學(PK)研究預計將在2H:21進行,並提供結果。

Milestones
里程碑
➢ HELP Topline data – June 2020
➢幫助拓撲數據-2020年6月
➢ End of Phase II Meeting with FDA – October 2020
➢結束與食品和藥物管理局的第二階段會議-2020年10月
➢ Acquisition of PHPM – January 2021
➢收購PHPM-2021年1月
➢ Finalize PH-HFpEF Phase III Trial Design – 2021
➢最終確定PH-HFpEF第三階段試驗設計-2021年
➢ PK and formulation work for imatinib in PAH – 2H:21
➢PK和伊馬替尼在PAH-2H:21中的配方工作
➢ Phase I trial for imatinib in PAH – 2H:21
伊馬替尼在多環芳烴-2H:21中的➢I期試驗
➢ Launch Phase III PH-HFpEF Trial – 1H:22
➢發射第三階段PH-HFpEF試驗-1H:22
➢ Site selection and enrollment for imatinib PH trial - 2022
2022年伊馬替尼PH試驗的➢選址和登記
➢ Launch Phase III in PH-HFpEF – 2022
➢在PH-HFpEF-2022年第三階段發射
➢ Imatinib PH trial topline report – 2024
2024年➢伊馬替尼PH試驗TOPLINE報告
➢ Completion of Phase III in PH-HFpEF - 2024
➢完成PH-HFpEF-2024年第三期工程
Summary
摘要
The HELP trial has ended and Tenax is now seeking regulatory guidance on next steps required to begin the Phase III portion of development. HELP's encouraging results have attracted further investment from Armistice Capital, a group that may catalyze additional funding to move forward into a pivotal study. Tenax has generated strong data for the PH-HFpEF indication with statistically significant results for the six minute walk test and other parameters that we think will be required in a Phase III. Based on the research and analysis included in our PAH update report, we believe PH-HFpEF patients will benefit from levosimendan's mechanism of action and clinical trials can be pursued with a reasonable cost and time commitment. The addition of PHPM and its Phase III ready asset drove our recent price increase along with the successful outcome of the HELP trial. Several near term objectives are expected to be completed for the imatinib program including FDA guidance and trial site identification which should support a 2022 start to the Phase III trial.
HELP試驗已經結束,Tenax現在正在就開始第三階段開發所需的下一步尋求監管指導。HELP的令人鼓舞的結果吸引了停戰資本(Armistice Capital)的進一步投資,該組織可能會催化額外的資金,以推進一項關鍵研究。Tenax已經為PH-HFpEF適應症產生了強有力的數據,6分鐘步行試驗和我們認為第三階段需要的其他參數的結果具有統計學意義。根據我們的PAH最新報告中包括的研究和分析,我們相信PH-HFpEF患者將受益於左西孟丹的作用機制,臨牀試驗可以在合理的成本和時間投入下進行。PHPM及其第三階段就緒資產的增加推動了我們最近的價格上漲以及HELP試驗的成功結果。伊馬替尼計劃的幾個近期目標預計將完成,包括FDA的指導和試驗地點的確定,這將支持2022年開始第三階段試驗。
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
訂閲Zacks Small CAP研究收到我們每天早上通過電子郵件直接發送給您的文章和報告。請訪問我們的網站有關Zacks SCR的更多信息,請訪問。
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
披露:Zacks SCR直接從發行人、從投資經理或從發行人聘請的投資者關係諮詢公司獲得了為期不少於一年的研究覆蓋範圍的補償。研究文章,如圖所示,是Zacks提供的服務的一部分,Zacks每季度收到這些服務的最高費用總計4萬美元。這裏有完整的免責聲明。
________________________
________________________
1. Fudim M, Boortz-Marx RL, Ganesh A, DeVore AD, Patel CB, Rogers JG, Coburn A, Johnson I, Paul A, Coyne BJ, Rao SV, Gutierrez JA, Kiefer TL, Kong DF, Green CL, Jones WS, Felker GM, Hernandez AF, Patel MR. Splanchnic Nerve Block for Chronic Heart Failure. JACC Heart Fail. 2020 Sep;8(9):742-752. doi: 10.1016/j.jchf.2020.04.010. Epub 2020 Jun 10. PMID: 32535123.
1.Fudim M,Boortz-Max RL,Ganesh A,DeVore AD,Patel CB,Rogers JG,Coburn A,Johnson I,Paul A,Coyne BJ,Rao SV,Gutierrez JA,Kiefer TL,Kong DF,Green CL,Jones WS,Felker GM,Hernandez AF,Patel Mr Patel Mr內臟神經阻滯治療慢性心力衰竭。JACC心力衰竭。2020年9月;8(9):742-752。Doi:10.1016/j.jchf.2020.04.010.EPub2020年6月10日。PMID:32535123。
2. Circulation that flows to the abdominal gastrointestinal organs including stomach, liver, spleen, pancreas, etc.
2.流向胃、肝、脾、胰腺等腹部胃腸器官的循環。
3. The 6 Minute Walk Test is a sub-maximal exercise test used to assess aerobic capacity and endurance. The distance covered over a time of 6 minutes is used as the outcome by which to compare changes in performance capacity.
3.6分鐘步行試驗是一種次極限運動試驗,用於評估有氧能力和耐力。在6分鐘的時間內覆蓋的距離被用作比較性能容量變化的結果。
4. Compiled by Zacks Analysts
4.由Zacks分析師彙編
5. Source: Tenax Therapeutics August 2021 Corporate Presentation
5.來源:Tenax Treeutics 2021年8月公司介紹