Mooning now
赞了
Mooning now
赞了
专栏OCGN
Let me reorganize my opinions for your reference.
1. Background information of OCGN
"Ocugen, Inc. is a biopharmaceutical company focused on discovering, developing, and commercializing gene therapies to cure blindness diseases and developing a vaccine to save lives from COVID-19." (According to official information, OCGN is specialized in developing, researching and commercializing (Biopharmaceutical companies that treat eye diseases and develop vaccines to save the world)
1.1 The relationship between OCGN and Covaxin
In fact, OCGN and Bharat Biotech signed an agreement in February 2021—according to the terms, Ocugen will have the patent rights for the vaccine (Covaxin) in the United States and Canada, and will be responsible for clinical development, regulatory approval (including EUA) and market development in the United States and Canada. commercialize. Bharat Biotech will also provide initial doses for use in the United States and Canada.
In addition, Bharat Biotech will support technology transfer for manufacturing in the United States and Canada. Taking into account the exclusive license for the US and Canadian markets, Ocugen will share the profits from the sale of COVAXIN in the US and Canadian markets with Bharat Biotech, and Ocugen will retain 45% of its profits.
OCGN has now submitted an EUA application to Canada, and the United States has submitted an IND application, laying the foundation for subsequent BLA applications.
2.Canada’s EUA
In September 2020, Health Canada passed a resolution to expedite Canada’s process of handling emergency vaccine use authorization (Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19), which was then changed to New Drug Submission for COVID-19. Applicants can apply to the authorities for EUA after all trials are over, and the authorities will also give priority to processing their applications.
The documents submitted by the applicant must meet the following conditions:
1. Pre-clinical studies (such as animal testing)
2. Clinical studies (all test results + safety data + valid data)
3. Manufacturing data (production data: vaccine production process)
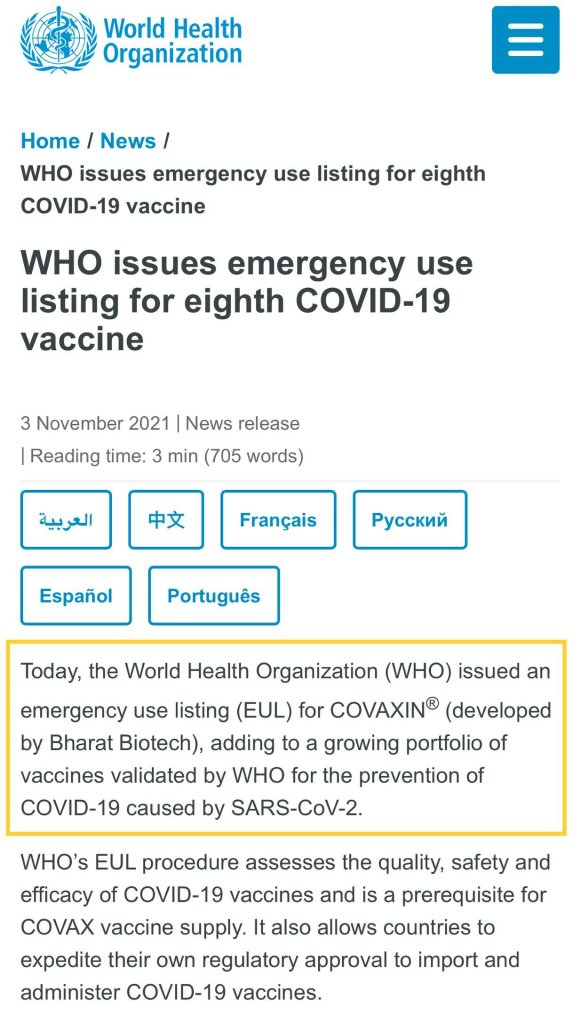
At present, the review of OCGN's EUA application in Canada through Vaccigen Ltd is still in progress. It coincides with the WHO just approved Covaxin's EUL application. Perhaps we can continue to wait and see.
3The relationship between WHO EUL and OCGN
In addition to EUA and FDA, some people have recently started to discuss WHO EUL. Let me explain briefly. WHO has formally approved Covaxin's EUL application on 3/11. In fact, Bharat Biotech applied directly to WHO for EUL. It seems that it has nothing to do with OCGN, but OCGN may benefit from it!
According to the agreement signed by OCGN and Bharat Biotech, one of the clauses deserves our attention: "if required under the Commercial Supply Agreement, and subject to any reasonable limitations on Ocugen's capacity (as more fully described in the Commercial Supply Agreement), Ocugen shall be responsible for the Manufacture and supply of the finished Product in its commercial packaging presentation, for use by BBIL in the Field in and for the BBIL Territory, after BBIL's receipt of Regulatory Approval for the Product in the BBIL Territory."
Simply put, OCGN can supply vaccines to Bharat Biotech after obtaining approval from the regulatory authorities (WHO/other countries) by Bharat Biotech (supply vaccines to regions outside of Canada and the United States). Assuming that Bharat Biotech obtains WHO EUL, Covaxin will join COVAX (Covid-19 Vaccine Implementation Plan) to assist in the distribution of vaccines to other countries (especially poor countries). And more countries/regions may expedite the approval of Covaxin's EUA application due to EUL (note that Bharat Biotech has previously submitted EUA to more than 60 countries/regions), and OCGN can take this opportunity to supply vaccines to other countries.
4. US BLA application (the first step of BLA application)
OCGN announced on 27/10 that it had submitted an Investigational New Drug Application (IND) to the US FDA to explore whether the effectiveness of Covaxin is applicable to the United States. This is also equivalent to OCGN starting to take the first step for the US BLA.
According to the information provided by OCGN, assuming that the IND application is successfully approved, OCGN will formally conduct clinical trials in the United States, and then compare whether the results of the trials in the United States and India are the same. OCGN said it hopes to complete the above tests in the first quarter of 2022. However, everyone should make it clear that this application is only an application for testing in the United States, not a formal application for the BLA.
According to FDA data, IND means that the drug has not been approved for sale/use in the United States. The FDA will approve the application within 30 days after receiving the IND application. If OCGN does not receive an objection from the FDA after 30 days, the trial can be conducted in the United States.
In conclusion, the IND application is the first step for OCGN to bring Covaxin to the United States. The above information also reflects that OCGN has started to prepare for the BLA. Let me add one point here. Among the many BLA application conditions, the local test results in the United States must be an important part of whether the FDA approves the BLA.
$Ocugen(OCGN.US$
1. Background information of OCGN
"Ocugen, Inc. is a biopharmaceutical company focused on discovering, developing, and commercializing gene therapies to cure blindness diseases and developing a vaccine to save lives from COVID-19." (According to official information, OCGN is specialized in developing, researching and commercializing (Biopharmaceutical companies that treat eye diseases and develop vaccines to save the world)
1.1 The relationship between OCGN and Covaxin
In fact, OCGN and Bharat Biotech signed an agreement in February 2021—according to the terms, Ocugen will have the patent rights for the vaccine (Covaxin) in the United States and Canada, and will be responsible for clinical development, regulatory approval (including EUA) and market development in the United States and Canada. commercialize. Bharat Biotech will also provide initial doses for use in the United States and Canada.
In addition, Bharat Biotech will support technology transfer for manufacturing in the United States and Canada. Taking into account the exclusive license for the US and Canadian markets, Ocugen will share the profits from the sale of COVAXIN in the US and Canadian markets with Bharat Biotech, and Ocugen will retain 45% of its profits.
OCGN has now submitted an EUA application to Canada, and the United States has submitted an IND application, laying the foundation for subsequent BLA applications.
2.Canada’s EUA
In September 2020, Health Canada passed a resolution to expedite Canada’s process of handling emergency vaccine use authorization (Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19), which was then changed to New Drug Submission for COVID-19. Applicants can apply to the authorities for EUA after all trials are over, and the authorities will also give priority to processing their applications.
The documents submitted by the applicant must meet the following conditions:
1. Pre-clinical studies (such as animal testing)
2. Clinical studies (all test results + safety data + valid data)
3. Manufacturing data (production data: vaccine production process)
At present, the review of OCGN's EUA application in Canada through Vaccigen Ltd is still in progress. It coincides with the WHO just approved Covaxin's EUL application. Perhaps we can continue to wait and see.
3The relationship between WHO EUL and OCGN
In addition to EUA and FDA, some people have recently started to discuss WHO EUL. Let me explain briefly. WHO has formally approved Covaxin's EUL application on 3/11. In fact, Bharat Biotech applied directly to WHO for EUL. It seems that it has nothing to do with OCGN, but OCGN may benefit from it!
According to the agreement signed by OCGN and Bharat Biotech, one of the clauses deserves our attention: "if required under the Commercial Supply Agreement, and subject to any reasonable limitations on Ocugen's capacity (as more fully described in the Commercial Supply Agreement), Ocugen shall be responsible for the Manufacture and supply of the finished Product in its commercial packaging presentation, for use by BBIL in the Field in and for the BBIL Territory, after BBIL's receipt of Regulatory Approval for the Product in the BBIL Territory."
Simply put, OCGN can supply vaccines to Bharat Biotech after obtaining approval from the regulatory authorities (WHO/other countries) by Bharat Biotech (supply vaccines to regions outside of Canada and the United States). Assuming that Bharat Biotech obtains WHO EUL, Covaxin will join COVAX (Covid-19 Vaccine Implementation Plan) to assist in the distribution of vaccines to other countries (especially poor countries). And more countries/regions may expedite the approval of Covaxin's EUA application due to EUL (note that Bharat Biotech has previously submitted EUA to more than 60 countries/regions), and OCGN can take this opportunity to supply vaccines to other countries.
4. US BLA application (the first step of BLA application)
OCGN announced on 27/10 that it had submitted an Investigational New Drug Application (IND) to the US FDA to explore whether the effectiveness of Covaxin is applicable to the United States. This is also equivalent to OCGN starting to take the first step for the US BLA.
According to the information provided by OCGN, assuming that the IND application is successfully approved, OCGN will formally conduct clinical trials in the United States, and then compare whether the results of the trials in the United States and India are the same. OCGN said it hopes to complete the above tests in the first quarter of 2022. However, everyone should make it clear that this application is only an application for testing in the United States, not a formal application for the BLA.
According to FDA data, IND means that the drug has not been approved for sale/use in the United States. The FDA will approve the application within 30 days after receiving the IND application. If OCGN does not receive an objection from the FDA after 30 days, the trial can be conducted in the United States.
In conclusion, the IND application is the first step for OCGN to bring Covaxin to the United States. The above information also reflects that OCGN has started to prepare for the BLA. Let me add one point here. Among the many BLA application conditions, the local test results in the United States must be an important part of whether the FDA approves the BLA.
$Ocugen(OCGN.US$

9
2
Mooning now
评论了
20
5
Mooning now
评论了
$BioNano Genomics(BNGO.US$ 为什么盘前上涨了3%?有人知道吗?
已翻译
1
6
Mooning now
评论了
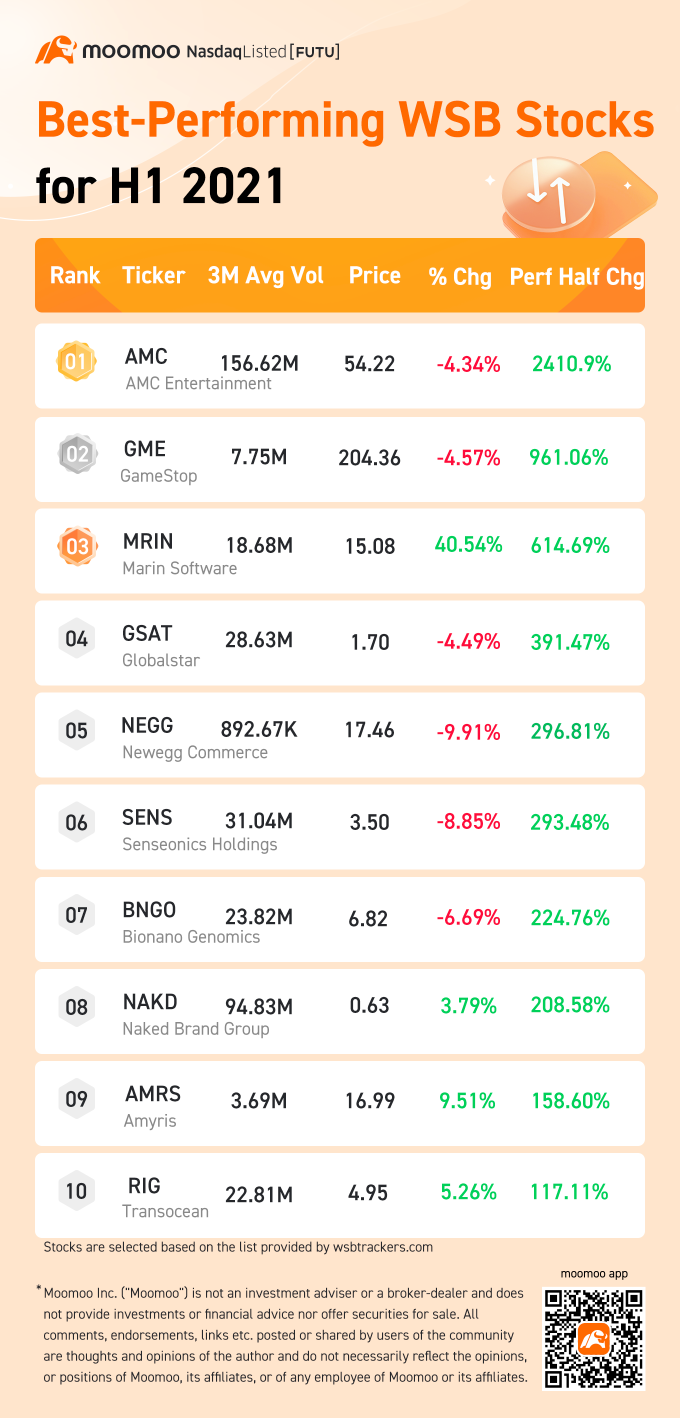
的股份 $游戏驿站(GME.US$和 $AMC院线(AMC.US$随着这两家公司继续受到散户投资者的青睐,2021年上半年一直在波动。
从GameStop开始,WallStreetBets的投资者已经成功地获得了在空头股数比例较高的股票中做空的名声。这股市场狂潮不仅引起了个人投资者的关注,也引起了对冲基金和美国证券交易委员会官员的注意。
从GameStop开始,WallStreetBets的投资者已经成功地获得了在空头股数比例较高的股票中做空的名声。这股市场狂潮不仅引起了个人投资者的关注,也引起了对冲基金和美国证券交易委员会官员的注意。
已翻译
89
22
Mooning now
评论了
$蔚来(NIO.US$ 上周有人问 49 美元能否进入,年初至今有人问 52 美元能否进入,今天有人再次问 54 美元能否进入。tmr 有人会再问 56 美元能否进入。😀😃😃🤣🤣😅😅😅 dyodd la。哈哈如果买入 liao drop den 逢低买入。截至目前支撑线是 42.8。
已翻译
10
14


![[empty]](https://static.moomoo.com/node_futunn_nnq/assets/images/folder.5c37692712.png)
![[error]](https://static.moomoo.com/node_futunn_nnq/assets/images/no-network.991ae8055c.png)