Exact Sciences' (EXAS) BLUE-C Study Results Published in NEJM
Exact Sciences Corporation EXAS recently announced the online publication of the BLUE-C study results in The New England Journal of Medicine (NEJM). The 20,000-participant, multi-center and prospective study was designed to determine the performance characteristics of the company’s next-generation Cologuard Plus multitarget stool DNA test.
The publication of the "Next-Generation Multitarget Stool DNA Test for Colorectal Cancer (CRC) Screening" peer-reviewed study in NEJM reflects a decade of Exact Sciences’ deep scientific and medical research in collaboration with Mayo Clinic.
Significance of the Cologuard Plus and BLUE-C Study
Exact Sciences and Mayo Clinic collaboratively developed Cologuard Plus, which features novel biomarkers and improved laboratory processes. It also incorporates enhanced sample stability components to provide patients more time to return their samples to EXAS' lab and increase the valid result rate. With CRC widely accepted as the most common and preventable cancer globally, the company is eager to bring an improved and noninvasive CRC screening test to patients in Cologuard Plus.
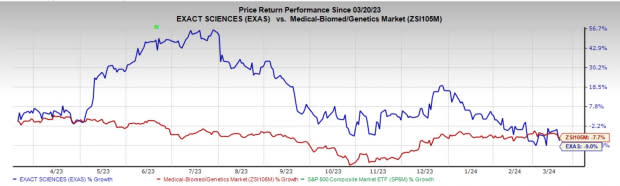
Image Source: Zacks Investment Research
One of the largest CRC screening trials ever conducted, the BLUE-C study design directly compared Cologuard Plus and an independent fecal immunochemical test (FIT), using colonoscopy as a reference method. The study investigators also collected blood samples for evaluation of a blood-based CRC screening test developed by Exact Sciences. Further, the study cohort was diverse and reflective of the population in the United States, which ensured that the BLUE-C findings and Cologuard Plus are relevant for all screen-eligible individuals, regardless of race or ethnicity.
The pre-market approval application for Cologuard Plus was submitted to the FDA in December 2023, and Exact Sciences plans to make the test available in 2025, pending approval.
More on the Study Outcome
The BLUE-C study results demonstrated that Cologuard Plus met all the endpoints, achieving 94% CRC sensitivity, 91% specificity including non-advanced findings, and 93% specificity including no findings. Specificity was even better in younger age groups, at 96% in the 45-54 year olds. Cologuard Plus will minimize unnecessary follow-up colonoscopies by reducing the likelihood of a false-positive screening test.
The outcome also showed that Cologuard Plus significantly outperformed an independent FIT for overall CRC sensitivity, treatable-stage CRC (stages I-III) sensitivity, high-grade dysplasia sensitivity and advanced precancerous lesion sensitivity. To potentially expand the eligible screening population beyond average-risk patients, the BLUE-C study enrolled a small subset of participants with a first-degree relative with a history of CRC.
Sensitivities for CRC and advanced precancerous lesions were similar among participants with a first-degree relative with a history of CRC and those without such a relative. Among the subset of nearly 19,000 participants who were average-risk, without a first-degree relative with a history of CRC, Cologuard Plus exhibited 95% sensitivity for CRC and 43% sensitivity for advanced precancerous lesions at a 91% specificity including non-advanced findings, or 94% specificity including no findings.
Industry Prospects
Per a Research report, the CRC screening market is likely to be worth $15.48 billion in 2024 and is expected to witness a CAGR of 5.16% by 2029.
Notable Highlights
According to Exact Sciences, Cologuard has become an essential part of healthcare providers’ screening tool kit partly because of patients’ preferences and demands. The adoption of the test is being driven by brand awareness and customer loyalty, helping to reach the 60 million Americans who are not up to date with colon cancer screening.
During fourth-quarter 2023, Exact Sciences continued to witness a broad-based momentum in the Cologuard adoption by healthcare providers, with an all-time high of 172,000 orders received for the screening test. Cologuard brand awareness reached an all-time high of 89%. Further, the company’s market research showed people who have never been screened prefer Cologuard 2 to 1 over colonoscopy.
Price Performance
In the past year, shares of EXAS have lost 9% compared with the industry’s decline of 7.7%.
Zacks Rank and Key Picks
Exact Sciences currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Cardinal Health (CAH), Stryker (SYK) and DaVita (DVA). While Cardinal Health and Stryker carry a Zacks Rank #2 (Buy), DaVita sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Cardinal Health’s stock has rallied 55.5% in the past year. Estimates for Cardinal Health’s fiscal 2024 earnings per share (EPS) have risen from $7.17 to $7.28 and from $7.94 to $8.03 in fiscal 2025 in the past 30 days.
CAH’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 15.6%. In the last reported quarter, it posted an earnings surprise of 16.67%.
Estimates for Stryker’s 2024 EPS have increased from $11.84 to $11.86 in the past 30 days. Shares of the company have moved 27.2% upward in the past year compared with the industry’s growth of 6.6%.
SYK’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 5.09%. In the last reported quarter, it delivered an average earnings surprise of 5.81%.
Estimates for DaVita’s 2024 EPS have moved from $8.86 to $8.97 in the past 30 days. Shares of the company have surged 75.3% in the past year compared with the industry’s 23.8% growth.
DVA’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 35.57%. In the last reported quarter, it delivered an average earnings surprise of 22.22%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Stryker Corporation (SYK) : Free Stock Analysis Report
DaVita Inc. (DVA) : Free Stock Analysis Report
Cardinal Health, Inc. (CAH) : Free Stock Analysis Report
Exact Sciences Corporation (EXAS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 