Novavax (NVAX) Q1 Earnings Miss, Stock Soars on Sanofi Deal
Shares of Novavax, Inc. NVAX skyrocketed nearly 99% on Friday after announcing a multi-billion-dollar deal with pharma giant Sanofi SNY for its protein-based COVID-19 vaccine, Nuvaxovid.
Sanofi Deal Breathes New Life
Beginning next year, Sanofi will gain rights to co-market Nuvaxovid globally, except in certain countries where Novavax has existing partnership agreements. The French drugmaker also has the sole license to develop and market the Novavax vaccine in combination with its influenza vaccine.
In return, Sanofi will make an upfront payment of $500 million to Novavax and up to $700 million in milestone payments, totaling up to $1.2 billion. Novavax will be eligible to receive tiered double-digit percentage royalty payments on sales by Sanofi of Nuvaxovid, Sanofi’s influenza-COVID combination vaccine and any other combination vaccines that Sanofi may develop, including Nuvaxovid.
However, both companies will still have the right to develop their own combined COVID-19-influenza combination vaccines and adjuvanted products at their own cost.
Sanofi will also have a non-exclusive license to use the company’s Matrix-M adjuvant technology in other vaccine products. In return, Novavax is entitled to receive a milestone payment of up to $200 million from Sanofi, plus mid-single-digit royalties.
In addition to the above deal, Novavax has also received an equity investment of nearly $70 million from Sanofi in exchange for a 4.9% stake.
This collaboration deal likely breathes new life for Novavax which had been facing uncertainties in its business for a long time. Despite being one of the handful of companies to have a marketed COVID-19 vaccine in its portfolio, it was not able to reap the benefits of the COVID-19 pandemic when compared with its peers like Moderna MRNA and Pfizer PFE.
As COVID-19 has ended, the demand for COVID-19 vaccines has fallen significantly. Though Moderna and Pfizer are also experiencing this significant decline across both their top- and bottom line, their profits generated from sales during the pandemic have helped them strengthen their cash position. This has allowed Moderna and Pfizer to make increased investments in R&D to not only expand their pipeline but also support their expected new product launches.
However, Novavax had been unable to enjoy these benefits as it had a delayed product launch thanks to some manufacturing issues which led to delayed regulatory filings and a delayed approval. In the last year, management had uncertainties about generating revenues from COVID-19 vaccine sales and raised concerns about its ability to continue as a ‘going concern.’ With the backing of a pharma bigwig like Sanofi, Novavax expects to increase the market share and presence of its COVID vaccine to a larger audience. This is likely one of the reasons that the company has now removed its ‘going concern’ warning.
Year to date, Shares of Novavax have surged 85.0% against the industry’s 6.6% fall.
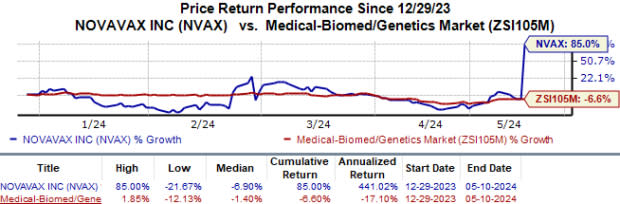
Image Source: Zacks Investment Research
Q1 Earnings & Sales Miss Estimates
In a separate press release, Novavaxreported a first-quarter 2024 loss of $1.05 cents per share, wider than the Zacks Consensus Estimate of a loss of $1.04. In the year-ago quarter, the company reported a loss of $3.41 per share.
Revenues in the quarter amounted to $94 million, also missing the Zacks Consensus Estimate of $101 million. However, the top line rose 16% on a year-over-year basis.
Quarter in Detail
In the reported quarter, the company recorded $82.3 million in product sales against a net reversal of $7.5 million in product sales in the year-ago period. The reported product sales missed our model estimates of $98 million.
During the quarter, management did not record any grant revenues. Management had recorded $87.4 million as grant revenues in the year-ago quarter.
Novavax recorded $11.5 million of revenues from royalties and adjuvant sales to licensing partners compared with the year-ago quarter’s revenues of $1.0 million.
In the reported quarter, research and development (R&D) expenses were $93 million, down almost 63% year over year. The downside can be attributed to a reduction in clinical and manufacturing spending during the quarter.
Selling, general and administrative (SG&A) expenses were down 23% year over year to $87 million, primarily due to the company's continued cost containment measures to reduce operating spend.
As of Mar 31, 2024, Novavax had $496 million of cash and cash equivalents compared with $584 million as of Dec 31, 2023.
2024 Guidance
Novavax now expects total revenues in the range of $400 million to $600 billion in 2024, nearly cutting down its previous guidance of $800 million to $1 billion by half.
However, the initial upfront payment of $500 million from Sanofi helps the company cover up for this curtailed revenue outlook.
The company expects its full-year combined R&D and SG&A expenses in the band of $700-$750 million, down from the previously issued guidance of $700-$800 million.
Recent Updates
Alongside the earnings result, management announced that it has aligned with the FDA on the path to securing an emergency use authorization (EUA) for its updated COVID-19 vaccine for the 2024-2025 vaccination season. It intends to make the product commercially available at the beginning of the season.
Novavax has also completed the submission of a regulatory filing with the FDA seeking full approval for its COVID-19 vaccine. This vaccine is currently authorized under the EUA pathway for use in individuals aged 12 years and older.
Management has made the strategic decision to add a stand-alone influenza arm to the company’s planned late-stage program on COVID-19-Influenza Combination (CIC) vaccine. The study will also focus on individuals at higher risk by enrolling older adults (aged 60 and older) for both stand-alone influenza and CIC arms of the study. It intends to start these studies in the second half of 2024, with the intent to market the same in 2026 commercially.
Management also claims to have been progressing well with its preclinical pipeline, which includes developing a new approach for vaccinating against H5N1 bird flu. It is also advancing core technology for different uses like mucosal vaccination and high-density nanoparticles.
Novavax, Inc. Price
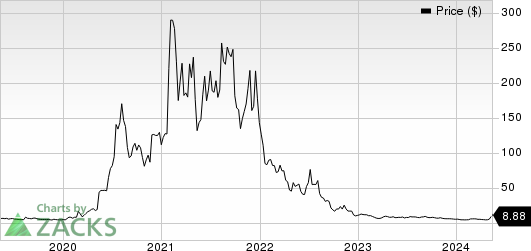
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 